Increasing Very Low-Dose Edoxaban Prescription: Effectiveness and Safety Data of Korean AF Patients
- PMID: 39601398
- PMCID: PMC11922598
- DOI: 10.4070/kcj.2024.0222
Increasing Very Low-Dose Edoxaban Prescription: Effectiveness and Safety Data of Korean AF Patients
Abstract
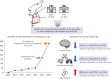
Background and objectives: Evidence remains limited on the real-world prescription of very low-dose oral anticoagulation among frail patients with atrial fibrillation (AF). We described the practice patterns, effectiveness, and safety of very low-dose edoxaban (15 mg once daily).
Methods: Patients with AF prescribed edoxaban 15 mg once daily in 2 tertiary hospitals between 2016 and September 2022 were included. Baseline clinical characteristics and clinical outcomes of interest were thromboembolic and bleeding events.
Results: A total of 674 patients were included (mean age 78.3±9.1, 49.7% aged ≥80 years, 49.3% women, median follow-up 1.0±1.2 years). Mean CHA2DS2-VASc score was 3.9±1.6, and the modified HAS-BLED score was 2.0±1.1. Between 2016 and 2022, the number of very low-dose edoxaban prescriptions increased. The main reasons for the prescription of very low-dose were low body weight (55.5% below 60 kg), anaemia (62.8%), chronic kidney disease (40.2%), active cancer (15.3%), concomitant anti-platelet use (26.7%), and prior major bleeding (19.7%). During a median follow-up duration of 8 (interquartile range 3-16) months, overall thromboembolic and bleeding events occurred in 16 (2.3%) and 88 (13.1%) patients, respectively. Compared to the expected event rates on the established risk scoring systems, patients receiving very low-dose edoxaban demonstrated a 61% reduction in ischemic stroke, a 68% reduction of ischemic stroke/transient ischemic attack/systemic embolism, whereas a 49% increase in major bleeding.
Conclusions: The prescription of very low-dose edoxaban was increased over time, attributable to various clinical factors. The use of very low-dose edoxaban reduced the expected risk of thromboembolic events.
Keywords: Atrial fibrillation; Bleeding; Factor Xa inhibitor; Off-label use; Stroke.
Copyright © 2025. The Korean Society of Cardiology.
Conflict of interest statement
So-Ryoung Lee: Speaking fees from Bayer, BMS/Pfizer, Biosense Webster, Daiichi-Sankyo, Sanofi-Aventis, Daewoong Pharmaceutical Co., Samjinpharm, Seers Technology, Biotronik, Boston Scientific and Medtronic. Consultant for Biosense Webster.
Figures



References
-
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2024;149:e1–e156. - PMC - PubMed
-
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. - PubMed
-
- Zhang XL, Zhang XW, Wang TY, et al. Off-label under- and overdosing of direct oral anticoagulants in patients with atrial fibrillation: a meta-analysis. Circ Cardiovasc Qual Outcomes. 2021;14:e007971. - PubMed
-
- Ruff CT, Giugliano RP, Braunwald E, et al. Association between edoxaban dose, concentration, anti-Factor Xa activity, and outcomes: an analysis of data from the randomised, double-blind ENGAGE AF-TIMI 48 trial. Lancet. 2015;385:2288–2295. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

