Human T cells engineered with an HLA-A2-restricted murine T-cell receptor targeting glypican 3 effectively control human hepatocellular carcinoma in mice
- PMID: 39601444
- PMCID: PMC12266797
- DOI: 10.1097/HEP.0000000000001175
Human T cells engineered with an HLA-A2-restricted murine T-cell receptor targeting glypican 3 effectively control human hepatocellular carcinoma in mice
Abstract
Background and aims: Glypican-3 (GPC3) is a promising target for T-cell therapy in HCC. While chimeric antigen receptor (CAR) T cells targeting GPC3 have demonstrated therapeutic efficacy, their effectiveness is limited by challenges such as low persistence and shedding of surface GPC3. Natural T-cell receptors (TCRs) may serve as an alternative, though identifying GPC3-specific TCRs within the endogenous repertoire is difficult.
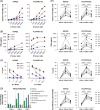
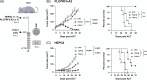
Approach and results: We immunized human leucocyte antigen-A2 (HLA-A2) transgenic mice with an adenovirus expressing human GPC3, identifying a panel of TCRs that recognize the GPC3(522-530) epitope. We cloned 3 murine GPC3-TCRs (TCR-A, TCR-B, and TCR-C) and engineered primary human T cells (TCR-T). TCR-T cells effectively recognized GPC3 + HLA-A2 + human HCC cells, with recognition diminished by GPC3 silencing and HLA-A2 blockade. TCR-B-T and TCR-C-T cells showed the highest reactivity, with TCR-B-T cells exhibiting superior effector functions, proliferative capacity, and therapeutic efficacy in xenograft HCC models. Notable, TCR-B-T cells outperformed second-generation 41BB GPC3-specific CAR-T cells, attributed to lower exhaustion, enhanced proliferation, greater effector function, and improved resilience. Furthermore, mixed dosing of CAR-T and TCR-B-T cells was significantly more effective than staggered dosing of the same cell type, suggesting potential synergistic effects.
Conclusions: Transgenic TCRs join forces with CARs, expanding the arsenal of GPC3-targeting receptors for HCC T-cell therapy.
Keywords: T-cell therapy; chimeric antigen receptor.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Bruno Sangro advises and is on the speakers’ bureau for AstraZeneca, Eisai, and Roche. He consults for or advises for Bayer, Incyte, and Boston Scientific. He advises BMS, MSD, Sanofi, and GSK. He is on the speakers’ bureau for Sirtex. The remaining authors have no conflicts to report.
Figures







References
-
- Shi D, Shi Y, Kaseb AO, Qi X, Zhang Y, Chi J, et al. Chimeric antigen receptor-glypican-3 t-cell therapy for advanced hepatocellular carcinoma: Results of phase I trials. Clin Cancer Res. 2020;26:3979–3989. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

