CaMKII-dependent non-canonical RIG-I pathway promotes influenza virus propagation in the acute-phase of infection
- PMID: 39601535
- PMCID: PMC11708044
- DOI: 10.1128/mbio.00087-24
CaMKII-dependent non-canonical RIG-I pathway promotes influenza virus propagation in the acute-phase of infection
Abstract
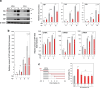
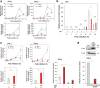
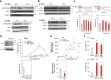
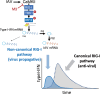
Ca2+/calmodulin-dependent protein kinase II (CaMKII) is one of hundreds of host-cell factors involved in the propagation of type A influenza virus (IAV), although its mechanism of action is unknown. Here, we identified CaMKII inhibitory peptide M3 by targeting its kinase domain using affinity-based screening of a tailored random peptide library. M3 inhibited IAV cytopathicity and propagation in cells by specifically inhibiting the acute-phase activation of retinoic acid-inducible gene I (RIG-I), which is uniquely regulated by CaMKII. Downstream of the RIG-I pathway activated TBK1 and then IRF3, which induced small but sufficient amounts of transcripts of the genes for IFN α/β to provide the capped 5'-ends that were used preferentially as primers to synthesize viral mRNAs by the cap-snatching mechanism. Importantly, knockout of RIG-I in cells almost completely inhibited the expression of IFN mRNAs and subsequent viral NP mRNA early in infection (up to 6 h after infection), which then protected cells from cytopathicity 24 h after infection. Thus, CaMKII-dependent acute-phase activation of RIG-I promoted IAV propagation, whereas the canonical RIG-I pathway stimulated antiviral activity by inducing large amounts of mRNA for IFNs and then for antiviral proteins later in infection. Co-administration of M3 with IAV infection rescued mice from the lethality and greatly reduced proinflammatory cytokine mRNA expression in the lung, indicating that M3 is highly effective against IAV in vivo. Thus, regulation of the CaMKII-dependent non-canonical RIG-I pathway may provide a novel host-factor-directed antiviral therapy.IMPORTANCEThe recent emergence of IAV strains resistant to commonly used therapeutic agents that target viral proteins has exacerbated the need for innovative strategies. Here, we originally identified CaMKII-inhibitory peptide M3, which efficiently inhibits IAV-lethality in vitro and in vivo. M3 specifically inhibited the acute-phase activation of RIG-I, which is a novel pathway to promote IAV propagation. Thus, this pathway acts in an opposite manner compared with the canonical RIG-I pathway, which plays essential roles in antiviral innate immune response later in infection. The CaMKII-dependent non-canonical RIG-I pathway can be a promising and novel drug target for the treatment of infections.
Keywords: CaMKII; RIG-I; cap-snatching; influenza virus; peptide library screening.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Simonsen L, Spreeuwenberg P, Lustig R, Taylor RJ, Fleming DM, Kroneman M, Van Kerkhove MD, Mounts AW, Paget WJ, GLaMOR Collaborating Teams . 2013. Global mortality estimates for the 2009 influenza pandemic from the GLaMOR project: a modeling study. PLoS Med 10:e1001558. doi: 10.1371/journal.pmed.1001558 - DOI - PMC - PubMed
-
- Tripathi S, Pohl MO, Zhou Y, Rodriguez-Frandsen A, Wang G, Stein DA, Moulton HM, DeJesus P, Che J, Mulder LCF, et al. 2015. Meta- and orthogonal integration of influenza “OMICs” data defines a role for UBR4 in virus budding. Cell Host & Microbe 18:723–735. doi: 10.1016/j.chom.2015.11.002 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 21H02629/MEXT | Japan Society for the Promotion of Science (JSPS)
- 21K19344/MEXT | Japan Society for the Promotion of Science (JSPS)
- Takeda Science Foundation (TSF)
- JP22ama121029j0001/Japan Agency for Medical Research and Development (AMED)
- JP21am0101093/Japan Agency for Medical Research and Development (AMED)
LinkOut - more resources
Full Text Sources
Miscellaneous

