Recombinant Saccharomyces cerevisiae EBY100/pYD1-FaeG: a candidate for an oral subunit vaccine against F4+ ETEC infection
- PMID: 39601541
- PMCID: PMC11784076
- DOI: 10.1128/aem.01817-24
Recombinant Saccharomyces cerevisiae EBY100/pYD1-FaeG: a candidate for an oral subunit vaccine against F4+ ETEC infection
Abstract
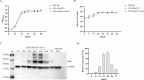
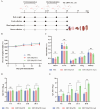
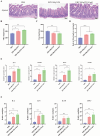
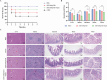
Diarrheal diseases attributable to multidrug-resistant F4+ enterotoxigenic Escherichia coli (ETEC) are escalating in severity, posing significant risks to the health and safety of both humans and animals. This study used Saccharomyces cerevisiae EBY100 to display the FaeG subunit of F4 colonizing factor as an oral vaccine against F4+ ETEC infection. Mice were orally immunized twice with 108 CFU of EBY100/pYD1-FaeG, followed by a challenge with F4+ ETEC EC6 on day 7 post-immunization. The results showed that the recombinant strain EBY100/pYD1-FaeG orally enhanced the growth of the small intestine villi, significantly boosted the expression of tight junction proteins (ZO-1, Occludin, MUC2, and Claudin) (P < 0.05), and modulated the gut microbiota composition. Additionally, immunization with EBY100/pYD1-FaeG also upregulated the levels of IL-2, IL-4, and IFN-γ in the intestines of mice (P < 0.01), while serum IgG and fecal sIgA titer significantly increased (P < 0.05). These immune responses enhanced the capacity to fight against ETEC, leading to an increased survival rate of mice and relieved damage to tissues and organs of mice infection. In summary, the study suggested that the recombinant Saccharomyces cerevisiae EBY100/pYD1-FaeG could effectively stimulate the immune response and generate specific antibodies against F4+ ETEC, showing its potential to serve as a subunit oral vaccine candidate for preventing F4+ ETEC infection.IMPORTANCEThe multidrug-resistant F4+ enterotoxigenic Escherichia coli (ETEC) strains are the primary clinical pathogens responsible for post-weaning diarrhea in piglets, resulting in substantial economic losses in the pig farming industry. In the study, we developed an oral vaccine candidate, Saccharomyces cerevisiae EBY100/pYD1-FaeG, to prevent diarrhea caused by multidrug-resistant F4+ ETEC. Oral administration of EBY100/pYD1-FaeG significantly enhanced immune responses, improved intestinal health, and provided protection against F4+ ETEC infection in mice. This approach offers a potential application prospect for preventing F4+ ETEC infections that lead to post-weaning diarrhea in clinical settings and provides a promising solution for addressing the growing threat of antibiotic resistance in bacterial pathogens.
Keywords: F4+ ETEC; Saccharomyces cerevisiae; oral vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
A tripartite fusion, FaeG-FedF-LT(192)A2:B, of enterotoxigenic Escherichia coli (ETEC) elicits antibodies that neutralize cholera toxin, inhibit adherence of K88 (F4) and F18 fimbriae, and protect pigs against K88ac/heat-labile toxin infection.Clin Vaccine Immunol. 2011 Oct;18(10):1593-9. doi: 10.1128/CVI.05120-11. Epub 2011 Aug 3. Clin Vaccine Immunol. 2011. PMID: 21813665 Free PMC article.
-
Immunogenicity of recombinant Lactobacillus casei-expressing F4 (K88) fimbrial adhesin FaeG in conjunction with a heat-labile enterotoxin A (LTAK63) and heat-labile enterotoxin B (LTB) of enterotoxigenic Escherichia coli as an oral adjuvant in mice.J Appl Microbiol. 2017 Feb;122(2):506-515. doi: 10.1111/jam.13352. Epub 2016 Dec 12. J Appl Microbiol. 2017. PMID: 27860074
-
Heterologous prime-boost immunization of two-component vaccine candidate PWDVax protected pigs against F18 enterotoxigenic Escherichia coli post-weaning diarrhea.Infect Immun. 2025 Apr 8;93(4):e0040624. doi: 10.1128/iai.00406-24. Epub 2025 Mar 12. Infect Immun. 2025. PMID: 40071919 Free PMC article.
-
Strategies to overexpress enterotoxigenic Escherichia coli (ETEC) colonization factors for the construction of oral whole-cell inactivated ETEC vaccine candidates.Appl Microbiol Biotechnol. 2012 Mar;93(6):2291-300. doi: 10.1007/s00253-012-3930-6. Epub 2012 Feb 16. Appl Microbiol Biotechnol. 2012. PMID: 22350259 Review.
-
Review on pathogenicity mechanism of enterotoxigenic Escherichia coli and vaccines against it.Microb Pathog. 2018 Apr;117:162-169. doi: 10.1016/j.micpath.2018.02.032. Epub 2018 Feb 21. Microb Pathog. 2018. PMID: 29474827 Review.
Cited by
-
Prevalence, Molecular Characterization, and Antimicrobial Resistance Profile of Enterotoxigenic Escherichia coli Isolates from Pig Farms in China.Foods. 2025 Mar 28;14(7):1188. doi: 10.3390/foods14071188. Foods. 2025. PMID: 40238372 Free PMC article.
References
-
- Kuhlmann FM, Laine RO, Afrin S, Nakajima R, Akhtar M, Vickers T, Parker K, Nizam NN, Grigura V, Goss CW, Felgner PL, Rasko DA, Qadri F, Fleckenstein JM. 2021. Contribution of noncanonical antigens to virulence and adaptive immunity in human infection with enterotoxigenic E. coli. Infect Immun 89:e00041-21. doi:10.1128/IAI.00041-21 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

