Effects of Subanesthetic Oromucosal Dexmedetomidine on Sleep in Humans: A Randomized, Controlled Pharmacokinetics-Pharmacodynamics Study
- PMID: 39601561
- PMCID: PMC11801451
- DOI: 10.1097/ALN.0000000000005314
Effects of Subanesthetic Oromucosal Dexmedetomidine on Sleep in Humans: A Randomized, Controlled Pharmacokinetics-Pharmacodynamics Study
Abstract
Background: The locus coeruleus noradrenergic system may provide a potential new target for pharmacologic insomnia treatment, particularly in patients suffering from elevated distress. The selective α 2 -noradrenergic agonist dexmedetomidine attenuates locus coeruleus activity in subanesthetic doses, yet no adequate nonparental delivery systems of dexmedetomidine are currently available. To examine the feasibility of oromucosal dexmedetomidine administration, the authors developed two distinct-one sublingual and one buccal-oromucosal, fast-disintegrating dexmedetomidine formulas tailored for self-administration. Here, the authors established the formulas' pharmacokinetic and pharmacodynamic profiles.
Methods: In a pilot study (sublingual formulation; n = 8 good sleepers) and a main study (buccal formulation; n = 17 poor sleepers), each following a randomized, double-blind, placebo-controlled crossover design, the authors investigated subanesthetic doses (20 and 40 µg) of the two formulas. They complemented the pharmacokinetic assessments with all-night polysomnography, nocturnal cortisol and melatonin measurements, assessments of cardiovascular functions during and after sleep, cortisol awakening response, and postawakening examination of subjective state and vigilance.
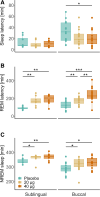
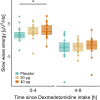
Results: Particularly buccal dexmedetomidine was rapidly absorbed and exhibited excellent dose proportionality with minimal between-subject variation in exposure. In poor sleepers, 40 µg buccal dexmedetomidine shortened the sleep latency by 11.5 min, increased the time spent in non-rapid eye movement sleep by 37.2 min, and elevated non-rapid eye movement sleep electroencephalographic slow-wave energy (0.75 to 4.0 Hz) in the first half of the night by roughly 23%. Rapid eye movement sleep latency was dose-dependently prolonged (20 µg, 55.0 min; 40 µg, 115.3 min). Nocturnal cortisol, melatonin and heart rate, and morning cortisol were not significantly affected by dexmedetomidine, nor did postawakening orthostatic regulation, subjective sleepiness and mood, and psychomotor vigilance differ among the conditions.
Conclusions: The favorable pharmacokinetic and pharmacodynamic profile of oromucosal dexmedetomidine delivery warrants further dose-finding and clinical studies to establish the exact roles of α 2 receptor agonism in pharmacologic sleep enhancement and as a possible novel mechanism to alleviate stress-related insomnia.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Society of Anesthesiologists.
Conflict of interest statement
Drs. Wespi, Dornbierer, and Landolt are listed as inventors on a pending patent on “Dexmedetomidine for the treatment of sleep disorders” (registration information: UniTectra Fall-Nr. UZ-231534a) that is assigned to University of Zurich, Zurich, Switzerland. Dr. Dornbierer declares that he cofounded Reconnect Labs, an academic spinoff company of the University of Zurich, focused on the development of dexmedetomidine-based products for the treatment of sleep disorders. Dr. Landolt has received consultation fees from Heel Biologische Heilmittel GmbH (Baden-Baden, Germany). The other authors declare no competing interests.
Figures

References
-
- Morin CM, Jarrin DC: Epidemiology of insomnia. Sleep Med Clin 2022; 17:173–91 - PubMed
-
- Van Someren EJW: Brain mechanisms of insomnia: New perspectives on causes and consequences. Physiol Rev 2021; 101:995–1046 - PubMed
-
- Holst SC, Landolt HP: Sleep-wake neurochemistry. Sleep Med Clin 2018; 13:137–46 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

