Tumor Expression of CD83 Reduces Glioma Progression and Is Associated with Reduced Immunosuppression
- PMID: 39601621
- PMCID: PMC11683667
- DOI: 10.1158/2767-9764.CRC-24-0281
Tumor Expression of CD83 Reduces Glioma Progression and Is Associated with Reduced Immunosuppression
Abstract
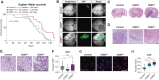
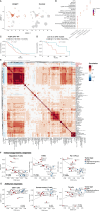
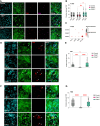
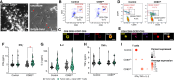
Abstract: Malignant glioma, the most lethal form of brain cancer, presents with an immunosuppressive microenvironment that obstructs tumor cell clearance and hampers immunotherapeutic interventions. Despite advancements in characterizing cellular and extracellular profiles in cancer, the immunosuppressive mechanisms specific to glioma remain poorly understood. We conducted single-cell RNA sequencing of glioma samples, which revealed a select subset of human and mouse glioma cells that express CD83, a marker associated with mature antigen-presenting cells. To investigate the impact of tumor cell CD83 expression on glioma outcomes, we used an immunocompetent mouse model of glioma, bioinformatic analyses of human samples, and in vitro assays. Our findings revealed that CD83+ tumor cells contribute to tumor growth suppression and are associated with enhanced cytotoxic T-cell profiles and activated CD8+ T cells. Increased proinflammatory cytokines were identified in CD83-overexpressing tumor conditions, which were also correlated with long-term CD8+ antitumor responses. Importantly, tumor-derived CD83 could mediate communication with T cells, altering the immune microenvironment to potentially enhance immune-related tumor clearance. Collectively, our data suggest that tumor cell expression of CD83 supports the endogenous antitumor T-cell constituency in malignant glioma. Future research endeavors may aim to further investigate whether CD83 expression can enhance immunotherapeutic approaches and improve patient outcomes.
Significance: Immunosuppression in malignant glioma remains a barrier to therapeutic development. CD83 overexpression in human and mouse glioma increases survival. CD83+ tumor cells promote signatures related to cytotoxic T cells, enhanced activation of CD8+ T cells, and increased proinflammatory cytokines. These findings suggest that tumor-expressed CD83 could mediate tumor-immune communications.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
C. Mohila reports ownership of Johnson & Johnson stock. Drugs and services provided by Johnson & Johnson are not used in this study. No other disclosures were reported.
Figures

References
-
- Mittelbronn M, Platten M, Zeiner P, Dombrowski Y, Frank B, Zachskorn C, et al. . Macrophage migration inhibitory factor (MIF) expression in human malignant gliomas contributes to immune escape and tumour progression. Acta Neuropathol 2011;122:353–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F99 CA274700/CA/NCI NIH HHS/United States
- S10 OD025240/OD/NIH HHS/United States
- R01 CA223388/CA/NCI NIH HHS/United States
- T32 HL092332/HL/NHLBI NIH HHS/United States
- P30 CA125123/CA/NCI NIH HHS/United States
- S10 RR024574/RR/NCRR NIH HHS/United States
- K00 CA274700/CA/NCI NIH HHS/United States
- R01 NS094615/NS/NINDS NIH HHS/United States
- F31 CA265156/CA/NCI NIH HHS/United States
- R35 NS132230/NS/NINDS NIH HHS/United States
- R01 NS124093/NS/NINDS NIH HHS/United States
- S10 OD023469/OD/NIH HHS/United States
- S10 OD025251/OD/NIH HHS/United States
- P30 EY002520/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

