Novel Uveal Melanoma Patient-Derived Organoid Models Recapitulate Human Disease to Support Translational Research
- PMID: 39601636
- PMCID: PMC11605663
- DOI: 10.1167/iovs.65.13.60
Novel Uveal Melanoma Patient-Derived Organoid Models Recapitulate Human Disease to Support Translational Research
Abstract
Purpose: A lack of representative human disease models has limited the translation of new and more effective treatments in uveal melanoma (UM), the most common primary adult intraocular malignancy. To fill this critical need, we developed and characterized a multicenter biobank of UM patient-derived organoids (PDOs).
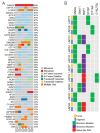
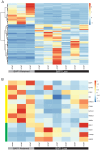
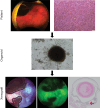
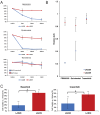
Methods: UM patients requiring enucleation from 2019 to 2024 donated tumor tissue for PDO generation. PDOs were cultured in Cultrex and compared to donor primary tumor using exome sequencing, RNA sequencing, and immunohistochemistry. The ability of PDOs to maintain the transformed phenotype was evaluated in an orthotopic xenograft model and monitored with fundus imaging. ATAC sequencing and drug response assays were done in a subset of PDOs to explore the feasibility of their use for mechanistic and translational studies.
Results: PDOs were successfully established in 40 of 44 cases (91%), retained clinically relevant mutations and molecular markers from the primary tumor, and displayed similar gene expression profiles and well-validated clinical prognostic markers of the disease. PDOs retained tumorigenic capacity in an in vivo model resembling human disease progression. Finally, we demonstrated that PDOs were a feasible platform to identify and evaluate novel therapeutic targets and investigate differential, personalized drug response.
Conclusions: PDO models offer a new platform with improved representation of human UM to aid in translational research for this dismal condition.
Conflict of interest statement
Disclosure:
Figures


References
-
- Singh AD, Turell ME, Topham AK.. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology. 2011; 118(9): 1881–1885. - PubMed
-
- Singh AD, Bergman L, Seregard S.. Uveal melanoma: epidemiologic aspects. Ophthalmol Clin North Am. 2005; 18(1): 75–84, viii. - PubMed
-
- Xu TT, Moser JC, Dalvin LA.. Uveal melanoma: laboratory advances and new frontiers in patient care. Curr Opin Ophthalmol. 2021; 32(3): 301–308. - PubMed
-
- Eskelin S, Pyrhönen S, Summanen P, Hahka-Kemppinen M, Kivelä T. Tumor doubling times in metastatic malignant melanoma of the uvea: tumor progression before and after treatment. Ophthalmology. 2000; 107(8): 1443–1449. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

