Impact of ursodeoxycholic acid treatment on Fontan-associated liver disease
- PMID: 39601803
- PMCID: PMC11794391
- DOI: 10.1007/s00535-024-02168-x
Impact of ursodeoxycholic acid treatment on Fontan-associated liver disease
Abstract
Background: Fontan-associated liver disease (FALD) is a type of progressive liver fibrosis that occurs following Fontan surgery and can be complicated by hepatocellular carcinoma (HCC). Established treatments for FALD are lacking. Therefore, we investigated the efficacy of ursodeoxycholic acid (UDCA) in patients with FALD.
Methods: This single-center retrospective study was conducted from 2003 to 2024 and involved 220 patients (103 men, 46.8%) who had been diagnosed with FALD. UDCA was administered to 113 patients presenting with liver or biliary enzyme abnormalities. We evaluated the patients' liver enzyme levels 3, 6, and 12 months after treatment. HCC developed in 10.5% and the mortality rate was 4.5%. Survival and cumulative incidence of HCC were compared between patients with and without UDCA treatment using Kaplan-Meier curves and propensity-matched analysis (n = 68 per group).
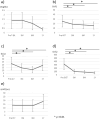
Results: UDCA treatment significantly reduced the aspartate aminotransferase (AST), alanine transaminase (ALT), and gamma-glutamyl transferase (GGT) levels at 3 months. The mean pretreatment AST/ALT/GGT levels were 26/22/323 U/L, respectively, and decreased to 19/15/102 U/L at 3 months, 18/12/88 U/L at 6 months, and 16/19/64 U/L at 12 months. However, the total bilirubin level and platelet count did not show significant differences. The survival rate was higher and the HCC rate was lower in patients with than without UDCA treatment. The 5-year incidence rate of HCC was 5.6% in the UDCA group and 24.2% in the untreated group.
Conclusions: UDCA treatment significantly reduced liver enzyme levels, including GGT, and mitigated the progression of HCC. UDCA may be beneficial for patients with FALD.
Keywords: Fontan procedure; Fontan-associated liver disease; Hepatocellular carcinoma; Ursodeoxycholic acid.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The author declare that they have no conflict of interest.
Figures



References
-
- Asrani SK, Asrani NS, Freese DK, et al. Congenital heart disease and the liver. Hepatology. 2012;56:1160–9. 10.1002/hep.25692. - PubMed
-
- Hilscher MB, Wells ML, Venkatesh SK, et al. Fontan-associated liver disease. Hepatology. 2022;75:1300–21. 10.1002/hep.32406. - PubMed
-
- Wu FM, Earing MG, Aboulhosn JA, et al. Predictive value of biomarkers of hepatic fibrosis in adult Fontan patients. J Heart Lung Transplant. 2017;36:211–9. 10.1016/j.healun.2016.07.011. - PubMed
-
- Elder RW, Parekh S, Book WM. More on hepatocellular carcinoma after the Fontan procedure. N Engl J Med. 2013;369:490. 10.1056/NEJMc1306854. - PubMed
-
- Téllez L, Payancé A, Tjwa E, et al. EASL-ERN position paper on liver involvement in patients with Fontan-type circulation. J Hepatol. 2023;79:1270–301. 10.1016/j.jhep.2023.07.013. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

