Valuation survey for SF-6Dv2 in Japan based on the international protocol
- PMID: 39602017
- PMCID: PMC11865146
- DOI: 10.1007/s11136-024-03830-w
Valuation survey for SF-6Dv2 in Japan based on the international protocol
Abstract
Purpose: The SF-6D Classification System was recently updated (SF-6Dv2). We performed a valuation survey to construct a value set for the SF-6Dv2 in Japan.
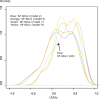
Methods: An online discrete choice experiment (DCE) with duration was used to estimate a value set for the SF-6Dv2 for Japan based on public preferences. The target sample number was 3800. Respondents were asked to complete 15 choice tasks. A conditional logit model that estimates interactions between time and each dimension was used to develop the value set.
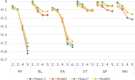
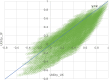
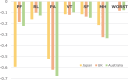
Results: The collected sample included 3933 respondents for the DCE tasks. The results of all the unconstrained models showed some inconsistencies. In particular, inconsistencies in the two most severe levels of the role limitation (RL) and vitality (VT) dimensions were observed in all models. The number of inconsistencies was smallest in a core model (n = 3) and in a model for core and common health states (n = 2). The physical functioning (PF) and pain (PA) dimensions had the greatest influence on utility at the overall level across all models. RL, VT, and social functioning (SF) had smaller overall impacts on utility. The PF weights for the two most severe levels are much lower than those in the UK and Australia. The Japanese scores tended to be lower compared with the UK SF-6Dv2 scores.
Conclusion: We obtained a value set for Japan (model 5). With the development of this value set, it is now possible to calculate quality-adjusted life years for economic evaluation in Japan when the SF-6Dv2 has been used.
Keywords: Health technology assessment; QALY; Quality of life; SF-6D; Utility.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflicts of interest: The authors have no conflicts of interests to declare that are relevant to the content of this article. Ethical approval: This study was performed in line with the principles of the Declaration of Helsinki. The ethics committee of the National Institute of Public Health, to which the first author belongs (NIPH-IBRA #12338) approved. Consent to participate: Informed consent was obtained from all individual participants included in the study. Consent for publication: Participants signed informed consent regarding publishing their anonymous data.
Figures
References
-
- National Institute of Health and Care Excellence (2022). NICE health technology evaluations: the manual. Retrieved November 14, 2024, from https://www.nice.org.uk/process/pmg36
-
- Shiroiwa, T. (2020). Cost-effectiveness evaluation for pricing medicines and devices: A new value-based price adjustment system in Japan. International Journal of Technology Assessment in Health Care,36(3), 270–276. 10.1017/s0266462320000264 - PubMed
-
- Center for Outcomes Research and Economic Evaluation for Health (C2H) (2024). Guideline for preparing cost-effectiveness evaluation to the central social insurance medical council. Retrieved November 14, 2024, from https://c2h.niph.go.jp/tools/guideline/guideline_en_2024.pdf
-
- Noto, S., Shiroiwa, T., Kobayashi, M., Murata, T., Ikeda, S., & Fukuda, T. (2020). Development of a multiplicative, multi-attribute utility function and eight single-attribute utility functions for the Health Utilities Index Mark 3 in Japan. Journal of Patient-Reported Outcomes,4(1), 23. 10.1186/s41687-020-00188-8 - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

