Liraglutide and Colesevelam Change Serum and Fecal Bile Acid Levels in a Randomized Trial With Patients With Bile Acid Diarrhea
- PMID: 39602188
- PMCID: PMC11596762
- DOI: 10.14309/ctg.0000000000000772
Liraglutide and Colesevelam Change Serum and Fecal Bile Acid Levels in a Randomized Trial With Patients With Bile Acid Diarrhea
Abstract
Introduction: Both liraglutide and colesevelam improve bile acid diarrhea symptoms. Colesevelam binds excess amounts of diarrhea-causing bile acids in the colon, whereas the mode of action for liraglutide remains elusive. In this article, we examined the impact of colesevelam and liraglutide treatment on the concentrations of bile acids in serum and feces and the fecal microbiota composition to better understand the 2 drugs' modes of action.
Methods: Bile acid species were analyzed in serum and fecal samples from a randomized, double-blind, double-dummy trial at baseline and after 3 and 6 weeks of orally administered colesevelam (1,875 mg twice daily, n = 26) or subcutaneously administered liraglutide (uptitrated by weekly increments of 0.6 mg from 0.6 to 1.8 mg daily, n = 26) in patients with 75 selenium-homotaurocholic acid test-verified, idiopathic, or postcholecystectomy bile acid diarrhea. Fecal microbiota composition was analyzed by 16S rRNA gene amplicon sequencing at the same time points.
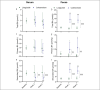
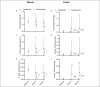
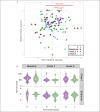
Results: Colesevelam increased the fecal concentrations of all bile acid species, whereas it decreased serum concentrations of secondary bile acids. Liraglutide induced a small increase in serum unconjugated bile acid concentrations without affecting fecal bile acid concentrations. No changes in fecal microbiota composition were observed with either treatment.
Discussion: Colesevelam and liraglutide exhibit distinct effects on serum and fecal bile acid concentrations with colesevelam reducing serum concentrations of secondary bile acids and promoting fecal bile acid excretion, whereas liraglutide enhances serum concentrations of unconjugated bile acids, potentially through deceleration of small intestinal transit time allowing more time for passive absorption of bile acids.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The American College of Gastroenterology.
Conflict of interest statement
Figures





References
-
- Wedlake L, A'Hern R, Russell D, et al. . Systematic review: The prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2009;30(7):707–17. - PubMed
-
- Hofmann AF, Mysels KJ. Bile salts as biological surfactants. Colloids Surf 1987;30(1):145–73.
-
- Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res 2006;47(2):241–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

