BCL2 regulates antibacterial autophagy in the intestinal epithelium
- PMID: 39602254
- PMCID: PMC11626146
- DOI: 10.1073/pnas.2410205121
BCL2 regulates antibacterial autophagy in the intestinal epithelium
Erratum in
-
Correction for Li et al., BCL2 regulates antibacterial autophagy in the intestinal epithelium.Proc Natl Acad Sci U S A. 2025 Dec 30;122(52):e2535281122. doi: 10.1073/pnas.2535281122. Epub 2025 Dec 24. Proc Natl Acad Sci U S A. 2025. PMID: 41439717 Free PMC article. No abstract available.
Abstract
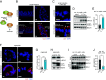
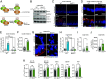
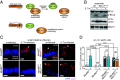
Autophagy is a key innate immune defense mechanism in intestinal epithelial cells. Bacterial invasion of epithelial cells activates antibacterial autophagy through a process that requires the innate immune adaptor protein MYD88, yet how MYD88 signaling connects to the autophagy machinery is unknown. Here, we show that the mouse intestinal pathogen Salmonella enterica Serovar Typhimurium (Salmonella Typhimurium) triggers MYD88 signaling that regulates binding of the anti-autophagy factor B cell lymphoma 2 (BCL2) to the essential autophagy protein Beclin1 (BECN1) in small intestinal enterocytes, a key epithelial cell lineage. Salmonella infection activated the kinase c-Jun N-terminal protein kinase 1 (JNK1) downstream of MYD88. JNK1 induced enterocyte BCL2 phosphorylation, promoting dissociation of the inhibitory BCL2-BECN1 complex and releasing BECN1 to initiate autophagy. Mice with BCL2 phosphorylation site mutations that prevent BCL2-BECN1 dissociation showed increased Salmonella invasion of enterocytes and dissemination to extraintestinal sites. These findings reveal that BCL2 links MYD88 signaling to enterocyte autophagy initiation, providing mechanistic insight into how invading bacteria trigger autophagy in the intestinal epithelium.
Keywords: autophagy; enterocyte; innate immunity; intestinal epithelium; pathogenic bacteria.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


References
-
- Thurston T. L. M., Ryzhakov G., Bloor S., Muhlinen N. v., Randow F., The TBK1 adaptor and autophagy receptor NDP52 restricts the proliferation of ubiquitin-coated bacteria. Nat. Immunol. 10, 1215–1221 (2009). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

