Macrophage subtypes inhibit breast cancer proliferation in culture
- PMID: 39602294
- PMCID: PMC11742110
- DOI: 10.1091/mbc.E24-06-0241
Macrophage subtypes inhibit breast cancer proliferation in culture
Abstract
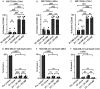
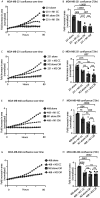
Macrophages are a highly plastic cell type that adopt distinct subtypes and functional states depending on environmental cues. These functional states can vary widely, with distinct macrophages capable of displaying opposing functions. We sought to understand how macrophage subtypes that exist on two ends of a spectrum influence the function of other cells. We used a coculture system with primary human macrophages to probe the effects of macrophage subtypes on breast cancer cell proliferation. Our studies revealed a surprising phenotype in which both macrophage subtypes inhibited cancer cell proliferation compared with cancer cells alone. Of particular interest, using two different proliferation assays with two different breast cancer cell lines, we showed that differentiating macrophages into a "protumor" subtype inhibited breast cancer cell proliferation. These findings are inconsistent with the prevailing interpretation that "protumor" macrophages promote cancer cell proliferation and suggest a re-evaluation of how these interpretations are made.
Figures



Update of
-
Macrophage subtypes inhibit breast cancer proliferation in culture.bioRxiv [Preprint]. 2024 Jun 1:2024.06.01.596963. doi: 10.1101/2024.06.01.596963. bioRxiv. 2024. Update in: Mol Biol Cell. 2025 Jan 1;36(1):br2. doi: 10.1091/mbc.E24-06-0241. PMID: 38853881 Free PMC article. Updated. Preprint.
References
-
- Cassetta L, Pollard JW (2020). Tumor-associated macrophages. Curr Biol 30, R246–R248. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

