Clinical impact of [18F]FDG-PET/CT in ARI0002h treatment, a CAR-T against BCMA for relapsed/refractory multiple myeloma
- PMID: 39602341
- PMCID: PMC11821407
- DOI: 10.1182/bloodadvances.2024014360
Clinical impact of [18F]FDG-PET/CT in ARI0002h treatment, a CAR-T against BCMA for relapsed/refractory multiple myeloma
Abstract
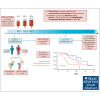
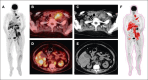
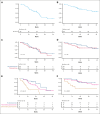
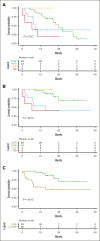
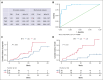
Multiple myeloma (MM) remains incurable, with poor outcomes in heavily pretreated patients. Chimeric antigen receptor (CAR) T-cell therapy has emerged as a promising treatment; however, outcomes after such therapy in patients with soft-tissue plasmacytomas and other bone lesions remain poorly understood. This study included 63 patients with relapsed/refractory MM treated either in the CARTBCMA-HCB-01 clinical trial (ARI0002h; academic B-cell maturation antigen [BCMA]-targeted CAR T-cell therapy) or in compassionate use. The aim was to evaluate the impact of soft-tissue involvement (extramedullary [EMD] and paraskeletal [PS] plasmacytomas) in response, survival and safety. Baseline [18F]fluorodeoxyglucose (FDG)-positron emission tomography (PET)/computed tomography (CT) from 5 participating centers were reviewed centrally. Of 63 patients, 52.4% presented plasmacytomas at the time of inclusion (21 PS, exclusively; and 12 EMD). Per responses, there were no significant differences between patients with and without plasmacytomas. A correlation was present between International Myeloma Working Group responses and those obtained by [18F]FDG-PET/CT at day 100 (Bologna criteria). No differences were observed in progression-free survival (PFS) or overall survival (OS) between patients with or without plasmacytomas. However, both PFS and OS were significantly shorter in patients with EMD. Interestingly, [18F]FDG-PET/CT response assessed on day 100, in accordance with the Bologna criteria, was predictive of survival outcomes. A metabolic tumor volume of ≥25 cm3 at baseline [18F]FDG-PET/CT was associated with earlier disease progression and shorter OS. These results highlight the importance of EMD evaluation by [18F]FDG-PET/CT before and after CAR T-cell infusion. This trial was registered at www.ClinicalTrials.gov as #NCT04309981; and EudraCT, 2019-001472-11.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: A.O.-C. declares nonfinancial support from Janssen, outside from the submitted work. V.C. received honoraria for lectures, consulting, and advisory boards from Johnson&Johnson, Bristol Myers Squibb, Sanofi, Amgen, Glaxo, Pfizer, BioGene, and Menarini; and received travel grants from Johnson&Johnson, Bristol Myers Squibb, Amgen, and BioGene; served on a speakers bureau for Bristol Myers Squibb; and reports financing of scientific research from Janssen. L.G.R.-L. declares honoraria and travel grants from Janssen, Amgen, Bristol Myers Squibb, GlaxoSmithKline, Menarini, and Sanofi. C.F.L. declares receiving grants through his institution from Bristol Myers Squibb, Janssen, and Amgen; reports honoraria from Amgen, Janssen, Bristol Myers Squibb, GlaxoSmithKline, and Sanofi; reports support for attending meetings or travel from Janssen, Bristol Myers Squibb, GlaxoSmithKline, and Amgen; and reports participation on advisory boards with Janssen, Bristol Myers Squibb, Amgen, Pfizer, and Sanofi. The remaining authors declare no competing financial interests.
Figures

References
-
- Moreau P, Kumar SK, San Miguel J, et al. Treatment of relapsed and refractory multiple myeloma: recommendations from the International Myeloma Working Group. Lancet Oncol. 2021;22(3):e105–e118. - PubMed
-
- Oliver-Caldés A, González-Calle V, Cabañas V, et al. Fractionated initial infusion and booster dose of ARI0002h, a humanised, BCMA-directed CAR T-cell therapy, for patients with relapsed or refractory multiple myeloma (CARTBCMA-HCB-01): a single-arm, multicentre, academic pilot study. Lancet Oncol. 2023;24(8):913–924. - PubMed
-
- Berdeja JG, Madduri D, Usmani SZ, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398(10297):314–324. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

