Comparable outcomes after busulfan- or treosulfan-based conditioning for allo-HSCT in children with ALL: results of FORUM
- PMID: 39602342
- PMCID: PMC11869852
- DOI: 10.1182/bloodadvances.2024014548
Comparable outcomes after busulfan- or treosulfan-based conditioning for allo-HSCT in children with ALL: results of FORUM
Abstract
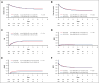
The superiority of total body irradiation (TBI)-based vs chemotherapy conditioning for allogeneic hematopoietic stem cell transplantation (allo-HSCT) in children with acute lymphoblastic leukemia (ALL) has been established in the international, prospective phase-3 FORUM study, randomizing 417 patients aged 4-18 years in complete remission (CR), who received allo-HSCT from HLA-matched sibling or unrelated donors. Because of the unavailability of TBI in some regions and to accommodate individual contraindications, this study reports the prespecified comparison of outcomes of patients receiving busulfan (BU)- or treosulfan (TREO)-based regimens from 2013 to 2018. Overall, 180 and 128 patients received BU/thiotepa (THIO)/fludarabine (FLU) or TREO/THIO/FLU, respectively. Data were analyzed as of February 2023, with a median follow-up of 4.2 years (range, 0.3-9.1). 3-year overall survival was 0.71 (BU, 95% confidence interval [0.64-0.77]) and 0.72 (TREO, [0.63-0.79]) and 3-year event-free survival was 0.60 (BU, [0.53-0.67]) and 0.55 (TREO, [0.46-0.63]). The 3-year cumulative incidence of relapse (BU, 0.31 [0.25-0.38]; TREO, 0.36 [0.27-0.44]); and nonrelapse mortality (BU, 0.08 [0.05-0.13]; TREO, 0.09 [0.05-0.15]) were comparable. One case of fatal veno-occlusive disease occurred in each group. No significant differences in acute and chronic graft-versus-host disease (GVHD) or 3-year GVHD-free and relapse-free survival (BU, 0.48 [0.41-0.55]; TREO, 0.45 [0.37-0.54]) were recorded. Outcomes for patients in first and second CR were similar irrespective of the regimen. In conclusion, BU/THIO/FLU or TREO/THIO/FLU regimens can be an alternative to TBI for patients with ALL aged >4 years with contraindications or lack of access to TBI. This trial was registered at www.ClinicalTrials.gov as #NCT01949129.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: K. Kalwak discloses speakers bureau fees from Novartis, medac, and Pierre Fabre; and reports travel grants from Pierre Fabre. P.B. reports travel grants from medac, Novartis, Vertex; and speakers bureau fees from medac, Novartis, Amgen. K. Kleinschmidt serves on advisory boards for Jazz and Vertex Pharmaceuticals; reports writing support grant from Jazz; and reports travel grants from Sobi. J.-H.D. reports consultancy with, and honoraria from, Orchard, Pierre Fabre Medicaments, Novartis, Pfizer, Vertex, and SymbioPharm; reports travel grants from Pierre Fabre Medicaments; and is a current equity holder in the private company Teva. A.B. reports speakers bureau fees from Novartis, Amgen, medac, and Neovii; and reports meeting support from medac and Neovii. H.P. reports travel grants from Jazz Pharmaceuticals and Neovii. T.G. serves on the advisory board for Novartis, Jazz, and Merck Sharp & Dohme; and reports travel grants from Neovii. J.B. reports advisory board participation for Janssen, Pfizer, and Novartis; is a study steering committee member with Novartis; and discloses speakers bureau fees from Novartis. C.P. reports study support from Sanofi; reports travel grants, study support and speakers bureau fees from Neovii. F.L. reports speakers bureau fees from Amgen, Bristol Myers Squibb, Miltenyi Biotec, Neovii, Novartis, Sobi, and Vertex; consultancy for Sanofi, Vertex, and Amgen; and reports honoraria from, and advisory board participation for, Amgen. The remaining authors declare no competing financial interests.
Figures


References
-
- Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295–304. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

