Susceptibility to and severity of SARS-CoV-2 infection according to prescription drug use-an observational study of 46,506 Danish healthcare workers
- PMID: 39602471
- PMCID: PMC11602038
- DOI: 10.1371/journal.pone.0311260
Susceptibility to and severity of SARS-CoV-2 infection according to prescription drug use-an observational study of 46,506 Danish healthcare workers
Abstract
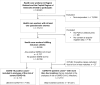
It is not well investigated whether exposure to specific drug classes is associated with COVID-19. We investigated the risk of SARS-CoV-2 infection and severe COVID-19 among healthcare workers according to prescription drug use. We conducted an observational study among Danish healthcare workers. SARS-CoV-2 positivity was defined as a positive PCR/ELISA test throughout 2020 and severe COVID-19 as any above 48-hour hospitalization within 14 days after infection. Patient characteristics came from online surveys while data on SARS-CoV-2, drugs and hospitalizations came from Danish Health Registers. Infected individuals were matched with uninfected controls based on age, sex, and chronic diseases. Drug exposure was defined as any prescription redemption in the past six and one month(s) before infection for each drug class. Models assessing the risk of infection (conditional logistic regression) and severe COVID-19 (logistic regressions) versus drug usage were adjusted for BMI, smoking, alcohol, education, region, and patient contact when possible. We matched 5,710 SARS-CoV-2-infected cases with 57,021 controls. The odds of infection were reduced by calcium channel blocker (adjusted odds ratio (aOR) 0.81, 95% Confidence Interval (CI): 0.66-1.00) and vasoprotective drug (aOR 0.77, CI: 0.62-0.95) usage during the six months before infection compared to no usage. Exposure to antibacterials in the past month increased the odds of infection (aOR 1.27, CI: 1.09-1.48). Among infected participants, the odds of severe COVID-19 were higher with usage of almost any investigated drug, especially, diuretics (crude odds radio (OR) 4.82, CI:2.15-10.83), obstructive airway disease drugs (OR 4.49, CI: 2.49-8.08), and antibacterials (OR 2.74 CI:1.62-4.61). In conclusion, antibacterials were associated with more SARS-CoV-2 infections and calcium channel blockers with less. Once infected, users of prescription drugs had higher odds of developing severe COVID-19. These findings suggest a need for studies to clarify interactions between specific drug groups, behaviour, known risk factors, and disease susceptibility/severity.
Copyright: © 2024 Eiken et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
S.B.’s research salary was sponsored by Sygesikring Danmark and the Capital Region of Denmark’s Research Foundation. K.I. received grants from Lundbeck Foundation to his institution [grant number R349-2020-731]. A.D.K has received a grant from The Danish Heart Foundation and a grant from the European Commission (EU 7th Framework [grant number 603266]). C.T-P. has received grants from Bayer and Novo- Nordisk for studies not related to the current study. F.F. has received research grant from the Novo Nordisk Foundation and an unrestricted research grant from the Laerdal Foundation to the Copenhagen EMS. S.D.N received an unrestricted research grants from the Novo Nordic Foundation, Augustinus Foundation, Kirsten and Freddy Johansen’s Foundation. U.F.R.’s research salary was sponsored by a grant from Kirsten and Freddy Johansen’s Foundation. A.E., M.V., H.B., J.R., R.B.H., J.H.K., P.B.N., M.M.P-H., K.F., J.B.N., O.A., T.K.F., R.B.D., S.R.O., S.B.D., M.G-B., E.S., L.H.H., T.B., F.N.E., H.E.P., H.U., and J.R. have no conflicts of interest to declare.
Figures


References
-
- Center JHUMCR. COVID-19 Dashboard 2022. [Available from: https://coronavirus.jhu.edu/map.html] n.d.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

