Inflammatory cytokines disrupt astrocyte exosomal HepaCAM-mediated protection against neuronal excitotoxicity in the SOD1G93A ALS model
- PMID: 39602529
- PMCID: PMC11601204
- DOI: 10.1126/sciadv.adq3350
Inflammatory cytokines disrupt astrocyte exosomal HepaCAM-mediated protection against neuronal excitotoxicity in the SOD1G93A ALS model
Abstract
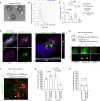
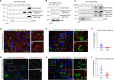
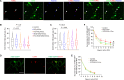
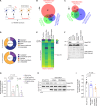
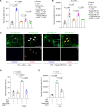
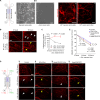
Astrocyte secreted signals substantially affect disease pathology in neurodegenerative diseases. It remains little understood about how proinflammatory cytokines, such as interleukin-1α/tumor necrosis factor-α/C1q (ITC), often elevated in neurodegenerative diseases, alter astrocyte-secreted signals and their effects in disease pathogenesis. By selectively isolating astrocyte exosomes (A-Exo.) and employing cell type-specific exosome reporter mice, our current study showed that ITC cytokines significantly reduced A-Exo. secretion and decreased spreading of focally labeled A-Exo. in diseased SOD1G93A mice. Our results also found that A-Exo. were minimally associated with misfolded SOD1 and elicited no toxicity to mouse spinal and human iPSC-derived motor neurons. In contrast, A-Exo. were neuroprotective against excitotoxicity, which was completely diminished by ITC cytokines and partially abolished by SOD1G93A expression. Subsequent proteomic characterization of A-Exo. and genetic analysis identified that surface expression of glial-specific HepaCAM preferentially mediates A-Exo's axon protection effect. Together, our study defines a cytokine-induced loss-of-function mechanism of A-Exo. in protecting neurons from excitotoxicity in amyotrophic lateral sclerosis.
Figures

References
-
- Nave K. A., Myelination and support of axonal integrity by glia. Nature 468, 244–252 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

