Effect of a Reduced PCV10 Dose Schedule on Pneumococcal Carriage in Vietnam
- PMID: 39602629
- PMCID: PMC11661757
- DOI: 10.1056/NEJMoa2400007
Effect of a Reduced PCV10 Dose Schedule on Pneumococcal Carriage in Vietnam
Abstract
Background: After pneumococcal disease and colonization have been controlled through vaccination campaigns, a reduced pneumococcal conjugate vaccine (PCV) schedule may be sufficient to sustain that control at reduced costs.
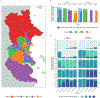
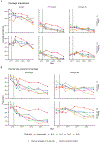
Methods: We investigated whether a single primary dose and booster dose (1p+1) of the 10-valent PCV (PCV10) would be noninferior to alternative dose schedules in sustaining control of carriage of pneumococcal serotypes included in the vaccine. In Nha Trang, Vietnam, an area in which PCV had not been used previously, a PCV10 catch-up campaign was conducted in which the vaccine was offered to children younger than 3 years of age, after which a cluster-randomized trial was conducted in which children received PCV10 at 2, 3, and 4 months of age (3p+0 group); at 2, 4, and 12 months of age (2p+1 group); at 2 and 12 months of age (1p+1 group); or at 12 months of age (0p+1 group). Annual carriage surveys in infants (4 to 11 months of age) and toddlers (14 to 24 months of age) were conducted from 2016 through 2020. The primary end point was protection against carriage of vaccine serotypes, evaluated in a noninferiority analysis in the 1p+1 group as compared with the 2p+1 and 3p+0 groups, 3.5 years after vaccine introduction (noninferiority margin, 5 percentage points). Noninferiority of the 0p+1 schedule was also evaluated.
Results: In 2016, before the introduction of PCV10, vaccine-serotype carriage was found in 160 of 1363 infants (11.7%); in 2020, vaccine-serotype carriage was found in 6 of 333 (1.8%), 5 of 340 (1.5%), and 4 of 313 (1.3%) infants in the 1p+1, 2p+1, and 3p+0 groups, respectively, indicating noninferiority of 1p+1 to 2p+1 (difference, 0.3 percentage points; 95% confidence interval [CI], -1.6 to 2.2) and to 3p+0 (difference, 0.5 percentage points; 95% CI, -1.4 to 2.4). Similarly, 1p+1 was noninferior to 2p+1 and 3p+0 for protection against vaccine-serotype carriage among toddlers. In 2016, carriage of serotype 6A was found in 99 of 1363 infants (7.3%); in 2020, it was found in 12 of 333 (3.6%), 10 of 340 (2.9%), and 3 of 313 (1.0%) infants in the 1p+1, 2p+1, and 3p+0 groups, respectively. The 0p+1 schedule was also noninferior to the other three dose schedules among infants and toddlers, although cross-protection against serotype 6A was less common than with the other vaccination schedules. No PCV10-associated severe adverse effects were observed.
Conclusions: A reduced vaccination schedule involving a single primary dose and booster dose of PCV10 was noninferior to alternative schedules in protecting against vaccine-serotype carriage in infants and toddlers. (Funded by the Bill and Melinda Gates Foundation and others; ClinicalTrials.gov number, NCT02961231.).
Copyright © 2024 Massachusetts Medical Society.
Conflict of interest statement
CONFLICT OF INTEREST
KM and CS are investigators on a clinical research collaboration with Pfizer on PCV vaccination in Mongolia and are investigators on a Merck Investigator Studies Program grant funded by MSD on pneumococcal serotype epidemiology in children. EMD is currently employed by Pfizer. The other authors have no conflict of interest in conducting the study.
Figures



References
-
- Shiri T, Datta S, Madan J, et al. Indirect effects of childhood pneumococcal conjugate vaccination on invasive pneumococcal disease: a systematic review and meta-analysis. Lancet Glob Health 2017; 5: e51–59. - PubMed
-
- von Gottberg A, de Gouveia L, Tempia S, et al. Effects of vaccination on invasive pneumococcal disease in South Africa. N Engl J Med 2014; 371: 1889–99. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
