Modulation of stress-related behaviour by preproglucagon neurons and hypothalamic projections to the nucleus of the solitary tract
- PMID: 39603502
- PMCID: PMC11667184
- DOI: 10.1016/j.molmet.2024.102076
Modulation of stress-related behaviour by preproglucagon neurons and hypothalamic projections to the nucleus of the solitary tract
Abstract
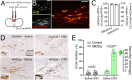
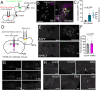
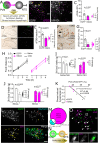
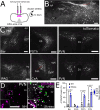
Stress-induced behaviours are driven by complex neural circuits and some neuronal populations concurrently modulate diverse behavioural and physiological responses to stress. Glucagon-like peptide-1 (GLP-1)-producing preproglucagon (PPG) neurons within the lower brainstem caudal nucleus of the solitary tract (cNTS) are particularly sensitive to stressful stimuli and are implicated in multiple physiological and behavioural responses to interoceptive and psychogenic threats. However, the afferent inputs driving stress-induced activation of PPG neurons are largely unknown, and the role of PPG neurons in anxiety-like behaviour is controversial. Through chemogenetic manipulations we reveal that cNTS PPG neurons have the ability to moderately increase anxiety-like behaviours in mice in a sex-dependent manner. Using an intersectional approach, we show that input from the paraventricular nucleus of the hypothalamus (PVN) drives activation of both the cNTS as a whole and PPG neurons in particular in response to acute restraint stress, but that while this input is rich in corticotropin-releasing hormone (CRH), PPG neurons do not express significant levels of receptors for CRH and are not activated following lateral ventricle delivery of CRH. Finally, we demonstrate that cNTS-projecting PVN neurons are necessary for the ability of restraint stress to suppress food intake in male mice. Our findings reveal sex differences in behavioural responses to PPG neural activation and highlight a hypothalamic-brainstem pathway in stress-induced hypophagia.
Keywords: Acute stress; Anxiety-like behaviour; Appetite; Corticotropin releasing hormone; Glucagon-like peptide-1; Nucleus of the solitary tract.
Copyright © 2024 The Authors. Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Ulrich-Lai Y.M., Herman J.P. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. http://www.nature.com/nrn/journal/v10/n6/suppinfo/nrn2647_S1.html - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

