Prognostic relevance of exercise pulmonary hypertension: results of the multicentre PEX-NET Clinical Research Collaboration
- PMID: 39603672
- PMCID: PMC11684422
- DOI: 10.1183/13993003.00698-2024
Prognostic relevance of exercise pulmonary hypertension: results of the multicentre PEX-NET Clinical Research Collaboration
Abstract
Background: Exercise pulmonary hypertension (PH) was defined by a mean pulmonary arterial pressure (mPAP)/cardiac output (CO) slope >3 mmHg·min·L-1 between rest and exercise in the 2022 European Society of Cardiology/European Respiratory Society PH guidelines. However, large, multicentre studies on the prognostic relevance of exercise haemodynamics and its added value to resting haemodynamics are missing.
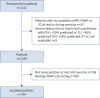
Patients and methods: The PEX-NET (Pulmonary Haemodynamics during Exercise Network) registry enrolled patients who underwent clinically indicated right heart catheterisations both at rest and ergometer exercise from 23 PH centres worldwide. In this retrospective analysis we included subjects with resting mPAP <25 mmHg and complete haemodynamic data at rest and exercise in the same body position. Mixed effects Cox proportional hazard models with random effect centre were applied to identify independent markers of prognosis among the haemodynamic parameters.
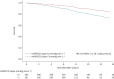
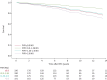
Results: We included 764 patients (64% females; median (interquartile range) age 59 (46-69) years and mPAP 17 (14-20) mmHg). Median (range) observation time was 6.8 (0.1-15.9) years and 87 patients (11%) died during follow-up. After adjustment for age, sex, haemoglobin level and resting haemodynamics, CO (hazard ratio (HR) 0.85, 95% CI 0.77-0.93; p=0.001) and transpulmonary gradient (HR 1.04, 95% CI 1.00-1.08; p=0.044) at peak exercise and the mPAP/CO slope (HR 1.12, 95% CI 1.06-1.18; p<0.001) were the only independent predictors of prognosis. Patients with a mPAP/CO slope >3 mmHg·min·L-1 had significantly worse survival compared to those with a mPAP/CO slope ≤3 mmHg·min·L-1 (HR 2.04, 95% CI 1.16-3.58; p=0.013).
Conclusion: The mPAP/CO slope is a robust and independent predictor of prognosis in patients with normal or mildly elevated resting PAP that provides prognostic information beyond resting haemodynamics and appears suitable to define exercise PH.
Copyright ©The authors 2024.
Conflict of interest statement
Conflict of interest: G. Kovacs reports support for the present study from the European Respiratory Society, grants from Janssen and Boehringer Ingelheim, consultancy fees from MSD, Boehringer Ingelheim, AOP Orphan, Chiesi, Ferrer, Bayer, Janssen, GSK, Liquidia and AstraZeneca, payment or honoraria for lectures, presentations, manuscript writing or educational events from MSD, Boehringer Ingelheim, AOP Orphan, Chiesi, Ferrer, Bayer, Janssen, GSK, Liquidia and AstraZeneca, support for attending meetings from MSD, Janssen, Boehringer Ingelheim and AOP Orphan, and participation on a data safety monitoring board or advisory board with MSD, Boehringer Ingelheim, Ferrer and Liquidia. M. Humbert reports grants from Gossamer and Merck, consultancy fees from 35 Pharma, Aerovate, AOP Orphan, Chiesi, Ferrer, Gossamer, Janssen, Keros, Liquidia, Merck, Respira and United Therapeutics, payment or honoraria for lectures, presentations, manuscript writing or educational events from Janssen and Merck, and participation on a data safety monitoring board or advisory board with 35 Pharma, Aerovate, Janssen, Keros, Merck and United Therapeutics. G.D. Lewis reports grants from the NIH and royalties from UpToDate. S. Ulrich reports grants from the Swiss National Science Foundation, MSD Switzerland, the Swiss Lung League, Gebro Swiss, Orpha Swiss and Janssen SA, consulting fees from MSD Switzerland, Gebro Swiss, Orpha Swiss and Janssen SA, payment for expert testimony from MSD Switzerland, Gebro Swiss, Orpha Swiss, Janssen SA and Novartis SA, and patents from MSD Switzerland, Gebro Swiss, Orpha Swiss and Janssen SA. R. Souza reports support for the present study from the European Respiratory Society, consulting fees from Bayer, Janssen and MSD, and payment or honoraria for lectures, presentations, manuscript writing or educational events from Bayer, Janssen and MSD. N. Galiè reports grants from Janssen and Merck, consultancy fees from Janssen, Merck and Actelion, payment or honoraria for lectures, presentations, manuscript writing or educational events from Janssen, Actelion, Chiesi and Ferrer, and support for attending meetings from Dompè. R. Malhotra reports support for the present study from NHLBI R01 HL159514, grants from the NHLBI, American Heart Association, Wild Family Foundation, Angea Biotherapeutics and Amgen, royalties from UpToDate and Pharmacologic BMP Inhibitors, and consulting fees from Renovacor, MyoKardia/BMS, Epizon Pharma and Third Pole. E. Grünig reports grants from Actelion, Janssen, Bayer, MSD, Merck, Ferrer, Acceleron, Actelion, Bayer, MSD, Janssen, Liquidia, United Therapeutics and OMT, consultancy fees from Actelion, Janssen, Bayer, MSD, Merck and Ferrer, payment or honoraria for lectures, presentations, manuscript writing or educational events from Actelion, Bayer/AOP, Janssen, phev, OMT, GEBRO, Ferrer and GWT, support for attending meetings from Janssen, participation on a data safety monitoring board or advisory board with MSD and Ferrer, and leadership roles with ADue (steering committee) and phev. B. Egenlauf reports payment or honoraria for lectures, presentations, manuscript writing or educational events from Janssen and MSD, payment for expert testimony from Bayer and AOP, and support for attending meetings from Janssen, MSD and OMT. R. Ewert reports consultancy fees from Boehringer Ingelheim, AOP Austria, Janssen Germany and AstraZeneca, payment or honoraria for lectures, presentations, manuscript writing or educational events from Berlin-Chemie Germany, Janssen Germany and Boehringer Germany, support for attending meetings from AOP Austria, Janssen Germany and Boehringer Ingelheim, and participation on a data safety monitoring board or advisory board with Janssen Germany and AOP Austria. A. Heine reports consultancy fees from TripleTre. R.J. Tedford reports consultancy fees from Abbott, Acorai, Aria CV Inc., Acceleron/Merck, Alleviant, Boston Scientific, Cytokinetics, Edwards LifeSciences, Endotronix, Gradient, Medtronic, Morphic Therapeutics, Restore Medical and United Therapeutics, support for attending meetings from Abiomed, participation on a data safety monitoring board or advisory board with Restore Medical, leadership roles with JHLT (Deputy Editor) and PH-LHD Task Force WSPH (Co-Chair), and stock (or stock options) with Aria CV. S. Rosenkranz reports grants from Actelion, AstraZeneca, Bayer, Janssen, Lempo and MSD, consulting fees from Abbott, Acceleron, Actelion, Aerovate, Altavant, AOP, AstraZeneca, Bayer, Ferrer, Gossamer, Janssen, Liquidia, MSD and UT, payment or honoraria for lectures, presentations, manuscript writing or educational events from Abbott, Actelion, AOP, AstraZeneca, Bayer, Boehringer Ingelheim, Edwards, Ferrer, Inari, Janssen, Lilly, MSD and UT, support for attending meetings from Bayer, participation on a data safety monitoring board or advisory board with Acceleron, Actelion, Aerovate, Altavant, AOP, Gossamer, Janssen, Liquidia, MSD and UT, and leadership as European Society of Cardiology Task Force chair in the 2022 European Society of Cardiology/European Respiratory Society guidelines on pulmonary hypertension. J.A. Barbera reports consultancy fees from Janssen-Cilag, MSD, Acceleron Pharma and Ferrer International, payment or honoraria for lectures, presentations, manuscript writing or educational events from Janssen-Cilag, MSD and Ferrer International, and support for attending meetings from Janssen-Cilag and MSD. I. Blanco reports consultancy fees from MSD, payment or honoraria for lectures, presentations, manuscript writing or educational events from MSD, Janssen and AOP Pharma, and support for attending meetings from MSD and Janssen. R.K.F. Oliveira reports grants from the National Council for Scientific and Technological Development (CNPq, Brazil; grants 313284/2021-0 and 409180/2022-0), consulting fees from Bayer Brazil and Janssen Brazil, payment or honoraria for lectures, presentations, manuscript writing or educational events from Bayer Brazil and Janssen Brazil, and support for attending meetings from Bayer Brazil. M. Andersen reports consultancy fees from Actelion, payment or honoraria for lectures, presentations, manuscript writing or educational events from Boehringer Ingelheim, and stock (or stock options) with Novo Nordisk, Eli Lilly and Zealand Pharma. L. Savale grants from MSD, payment or honoraria for lectures, presentations, manuscript writing or educational events from MSD, and Janssen and Janssen, support for attending meetings from MSD, and Janssen and Janssen. B.A. Maron reports grants from Deerfield Company, NIH, Cardiovascular Medical Research Education Foundation, consultancy fees from Regeneron, patents planned, issued or pending (US2019/059890, #9,605,047, PCT/US2020/066886), and participation on a data safety monitoring board or advisory board with Actelion Pharmaceuticals. R. Condliffe reports payment or honoraria for lectures, presentations, manuscript writing or educational events from Janssen, support for attending meetings from Janssen, and participation on a data safety monitoring board or advisory board with Boston Scientific. S. Mak reports support for the present study from Heart and Stroke Foundation of Canada, consultancy fees from AstraZeneca, participation on a data safety monitoring board or advisory board with University of Texas Southwestern, and a leadership role as Division Director Cardiology, University of Toronto. M. D'Alto reports consultancy fees from MSD, payment or honoraria for lectures, presentations, manuscript writing or educational events from MSD, Janssen, AOP, Dompè and Ferrer, and support for attending meetings from Dompè and Ferrer. H. Olschewski reports consultancy fees from Actelion, Chiesi, AstraZeneca, GSK, Bayer, Inventiva, Boehringer, Ferrer, Janssen, Menarini, MSD and Sanofi, payment or honoraria for lectures, presentations, manuscript writing or educational events from Springer, Medupdate and Mondial, support for attending meetings from Boehringer, Menarini and MSD, participation on a data safety monitoring board or advisory board with Aerovate, Bayer, Pfizer and IQVIA, receipt of equipment, materials, drugs, medical writing, gifts or other services from Boehringer, and the following financial (or non-financial) interests: Deputy Director Ludwig Boltzmann Institute for Lung Vascular Research, Graz, Austria. The remaining authors have no potential conflicts of interest to disclose.
Figures
Comment in
-
"To exercise or not to exercise," that is the question!Eur Respir J. 2024 Dec 19;64(6):2401947. doi: 10.1183/13993003.01947-2024. Print 2024 Dec. Eur Respir J. 2024. PMID: 39736110 No abstract available.
