Impact of switching to a dolutegravir-based regimen on body weight changes: insights from West African adult HIV cohorts
- PMID: 39604062
- PMCID: PMC11602402
- DOI: 10.1002/jia2.26371
Impact of switching to a dolutegravir-based regimen on body weight changes: insights from West African adult HIV cohorts
Abstract
Introduction: Adverse metabolic effects related to dolutegravir (DTG) are increasingly reported as countries are adopting DTG-based regimens as first-line antiretroviral therapy (ART), but there is limited data from sub-Saharan Africa. We explored changes in body weight pre- and post-switch to a DTG-based regimen and assessed the association between DTG switch and significant weight gain (SWG) defined as a ≥10% increase over a 12-month period in people living with HIV (PLHIV) on ART in West Africa.
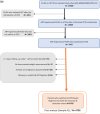
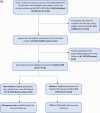
Methods: We first included all PLHIV followed in the IeDEA West Africa cohorts between January 2017 and June 2021, with a documented switch to DTG during 2019-2021 and in care ≥36 months at the day of switch. Weight change was estimated using a two slope piecewise linear mixed model with change point at the switch date. Secondly, we emulated a sequence of target trials (ETT) based on the observational data, performing pooled logistic regression analysis to compare SWG occurrence between PLHIV who switched to DTG and those who did not.
Results: We first included 6705 PLHIV from Burkina Faso, Côte d'Ivoire and Nigeria. Their median age at the time of switch was 48 years (IQR: 42-54) with a median follow-up of 9 years (IQR: 6-12), 63% were female. Most patients switched from efavirenz (EFV)-based ART (56.6%) and nevirapine (NVP)-based ART (30.9%). The overall post-switch annual average weight gain (AAWG) was significantly elevated at 3.07 kg/year [95% CI: 2.33-3.80] compared to the pre-switch AWG which stood at 0.62 kg/year [95% CI: 0.36-0.88]. The post-switch AWG was greater in patients previously on EFV and protease inhibitor (PI)-based ART compared to those on NVP-based ART. The pooled logistic regression analyses of a sequence of 24 ETT, including 9598 person-trials, switching to DTG was significantly associated with an SWG (aOR = 2.54; 95% CI = 2.18-2.97).
Conclusions: In West Africa, a 12-month DTG exposure was associated with substantial weight gain, especially in PLHIV previously on EFV and PI-based ARTs. Continuous weight monitoring and metabolic profiling is imperative in HIV cohorts to delineate the long-term cardiometabolic impact of DTG as patients with, or at elevated risk for cardiovascular diseases might benefit from alternative ART regimens.
Keywords: HIV; West Africa; antiretroviral therapy; dolutegravir; observational cohort study; weight gain.
© 2024 The Author(s). Journal of the International AIDS Society published by John Wiley & Sons Ltd on behalf of International AIDS Society.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Babatunde AO, Akin‐Ajani OD, Abdullateef RO, Togunwa TO, Isah HO. Review of antiretroviral therapy coverage in 10 highest burden HIV countries in Africa: 2015–2020. J Med Virol. 2023;95(1):e28320. - PubMed
-
- World Health Organization (WHO) . HIV/AIDS. Accessed 17 July 2023. Available at: https://www.who.int/news‐room/fact‐sheets/detail/hiv‐aids
-
- Kanters S, Vitoria M, Doherty M, Socias ME, Ford N, Forrest JI, et al. Comparative efficacy and safety of first‐line antiretroviral therapy for the treatment of HIV infection: a systematic review and network meta‐analysis. Lancet HIV. 2016;3(11):e510–e520. - PubMed
-
- Molina JM, Clotet B, van Lunzen J, Lazzarin A, Cavassini M, Henry K, et al. Once‐daily dolutegravir versus darunavir plus ritonavir for treatment‐naive adults with HIV‐1 infection (FLAMINGO): 96 week results from a randomised, open‐label, phase 3b study. Lancet HIV. 2015;2(4):e127–e136. - PubMed
-
- Deutschmann E, Bucher HC, Jaeckel S, Gibbons S, McAllister K, Scherrer AU, et al. Prevalence of potential drug–drug interactions in patients of the Swiss HIV cohort study in the era of HIV integrase inhibitors. Clin Infect Dis. 2021;73(7):e2145–e2152. - PubMed
MeSH terms
Substances
Grants and funding
- U01AI069919/CA/NCI NIH HHS/United States
- U01AI069919/HL/NHLBI NIH HHS/United States
- U01AI069919/AA/NIAAA NIH HHS/United States
- U54 AG062334/AG/NIA NIH HHS/United States
- U01AI069919/TW/FIC NIH HHS/United States
- U01 AI069919/AI/NIAID NIH HHS/United States
- U01AI069919/DA/NIDA NIH HHS/United States
- U01AI069919/DK/NIDDK NIH HHS/United States
- U01AI069919/National Institute of Allergy and Infectious Diseases
- U01AI069919/Eunice Kennedy Shriver National Institute of Child Health and Human Development
- U01AI069919/U.S. National Institutes of Health, National Institute of Allergy and Infectious Diseases
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

