Longitudinal Body Composition Identifies Hepatocellular Carcinoma With Cachexia Following Combined Immunotherapy and Target Therapy (CHANCE2213)
- PMID: 39604073
- PMCID: PMC11634469
- DOI: 10.1002/jcsm.13615
Longitudinal Body Composition Identifies Hepatocellular Carcinoma With Cachexia Following Combined Immunotherapy and Target Therapy (CHANCE2213)
Abstract
Background: Cancer cachexia can impact prognosis, cause resistance to anticancer treatments and affect the tolerability of treatments. This study aims to identify hepatocellular carcinoma (HCC) with cachexia by characterizing longitudinal body composition (BC) trajectories.
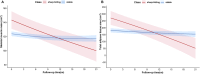
Methods: This longitudinal, multicentre cohort study included unresectable HCC patients treated with first-line programmed death-(ligand)1 inhibitors plus anti-vascular endothelial growth factor antibody/tyrosine kinase inhibitors between 01/2018-12/2022. BC measurements including skeletal muscle mass (SMM) and total adipose tissue area (TATA) were evaluated by computed tomography at the third lumbar vertebra at baseline and follow-up imaging. Unsupervised latent class growth mixed models were applied to distinguish potential longitudinal SMM and TATA trajectories for identifying cachexia. The primary study endpoint was overall survival (OS), with secondary endpoints including progression-free survival (PFS), objective response rate (ORR) and safety. Multiple Cox proportional hazards models were used to calculate adjusted hazard ratios (HRs) for survival.
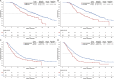
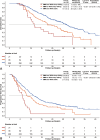
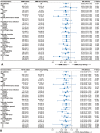
Results: A total of 411 patients with 2138 time-point measurements were included. The median age was 56 years, and 50 (12.2%) patients were female. Two distinct trajectories were identified for SMM and TATA: sharp-falling and stable. SMM sharply declined in 58 patients (14.1%) and TATA in 71 of 406 patients (17.5%) with significant worse OS (for SMM, 17.0 vs. 24.9 months; p < 0.001; HR = 0.59; for TATA, 15.3 vs. 25.1 months; p < 0.001; HR = 0.44). Patients were categorized into three phases based on trajectories: pre-cachexia (SMM and TATA stable, n = 299, 73.6%), cachexia (SMM or TATA sharp-falling, n = 86, 21.2%) and refractory cachexia (SMM and TATA sharp-falling, n = 21, 5.2%). Patients with refractory cachexia exhibited the worst OS, PFS and ORR, followed by those with cachexia. The median OS was 11.5 months for refractory cachexia, 17.7 for cachexia and 26.0 for pre-cachexia; median PFS was 6.0, 7.9 and 10.9 months, respectively, with ORR of 4.8%, 39.5% and 54.2%, respectively (all ps < 0.001). Multivariable Cox analysis identified refractory cachexia as an independent risk factor for both OS (HR = 3.31; p < 0.001) and PFS (HR = 2.94; p < 0.001), with cachexia also showing significant impacts. Grade 3-4 adverse events were higher in patients with refractory cachexia (23.8%) and cachexia (8.1%) compared with pre-cachexia (6.0%; p = 0.010).
Conclusions: HCC patients with cachexia and refractory cachexia were identified by longitudinal BC trajectories. Falling trajectories of BC identified refractory cachexia patients with worst response, survival and poor tolerability from systemic therapy combinations.
Trial registration: ClinicalTrials.gov identifier: NCT05278195.
Keywords: body composition; cachexia; hepatocellular carcinoma; immunotherapy; longitudinal trajectory; molecular targeted therapy.
© 2024 The Author(s). Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
Author's declaration of personal interests are as follows: Dr. Bernhard Scheiner received grant support from AstraZeneca and Eisai, speaker honoraria from Eisai and travel support from AbbVie, AstraZeneca, Ipsen and Gilead. Dr. David J. Pinato received lecture fees from ViiV Healthcare and Bayer Healthcare and travel expenses from BMS and Bayer Healthcare; consulting fees for Mina Therapeutics, EISAI, Roche, Astra Zeneca, DaVolterra, Exact Sciences, MURSLA, Avamune, BMS, LIfT Biosciences and Starpharma; and received research funding (to institution) from MSD, BMS and GSK. Dr. Fanpu Ji received lecture fees from Gilead Sciences, MSD and Ascletis; he is a consultant for Gilead, MSD. All other authors declare no conflicts of interest.
Figures
References
-
- Sung H., Ferlay J., Siegel R. L., et al., “Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries,” CA: A Cancer Journal for Clinicians 71 (2021): 209–249. - PubMed
-
- Finn R. S., Qin S., Ikeda M., et al., “Atezolizumab Plus Bevacizumab in Unresectable Hepatocellular Carcinoma,” New England Journal of Medicine 382 (2020): 1894–1905. - PubMed
-
- Ren Z., Xu J., Bai Y., et al., “Sintilimab Plus a Bevacizumab Biosimilar (IBI305) Versus Sorafenib in Unresectable Hepatocellular Carcinoma (ORIENT‐32): A Randomised, Open‐Label, Phase 2‐3 Study,” Lancet Oncology 22 (2021): 977–990. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
- 82130060/National Natural Science Foundation of China
- 82170626/National Natural Science Foundation of China
- 2018YFA0704100/National Key Research and Development Program
- 2018YFA0704104/National Key Research and Development Program
- CXZX202219/Jiangsu Provincial Medical Innovation Center
- xtr062023003/Fundamental Research Funds for the Central Universities
- Collaborative Innovation Center of Radiation Medicine of Jiangsu Higher Education Institutions
- 202205045/Nanjing Life Health Science and Technology Project
- YKK23268/Medical Science and Technology Development Fund Project of Nanjing
- KYCX21_0158/Postgraduate Research and Practice Innovation Program of Jiangsu Province
LinkOut - more resources
Full Text Sources
Medical
Research Materials

