Distinct Requirements for CD4+ T Cell Help for Immune Responses Induced by mRNA and Adenovirus-Vector SARS-CoV-2 Vaccines
- PMID: 39604225
- PMCID: PMC11739681
- DOI: 10.1002/eji.202451142
Distinct Requirements for CD4+ T Cell Help for Immune Responses Induced by mRNA and Adenovirus-Vector SARS-CoV-2 Vaccines
Abstract
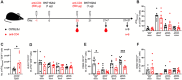
CD4+ T cells have been established as central orchestrators of cellular and humoral immune responses to infection or vaccination. However, the need for CD4+ T cell help to generate primary CD8+ T cell responses is variable depending on the infectious agent or vaccine and yet consistently required for the recall of CD8+ T cell memory responses or antibody responses. Given the deployment of new vaccine platforms such as nucleoside-modified mRNA vaccines, we sought to elucidate the requirement for CD4+ T cell help in the induction of cellular and antibody responses to mRNA and adenovirus (Ad)-vectored vaccines against SARS-CoV-2. Using antibody-mediated depletion of CD4+ T cells in a mouse immunization model, we observed that CD4+ T cell help was dispensable for both primary and secondary CD8+ T cell responses to the BNT162b2 and mRNA-1273 mRNA vaccines but required for the AZD1222 Ad-vectored vaccine. Nonetheless, CD4+ T cell help was needed by both mRNA and Ad-vectored vaccine platforms for the generation of antibodies, demonstrating the centrality of CD4+ T cells in vaccine-induced protective immunity against SARS-CoV-2. Ultimately, this highlights the shared and distinct regulation of humoral and cellular responses induced by these vaccine platforms.
Keywords: CD4 T cell help; CD8 T cells; T cell memory; T cell priming; adenovirus vaccines; antibodies; mRNA vaccines; vaccination.
© 2024 The Author(s). European Journal of Immunology published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors note the following conflicts of interest: N.M.P. has received consulting fees from Infinitopes. P.K. has received consulting fees from UCB, Biomunex, AstraZeneca, and Infinitopes. E.B. consults for AstaZeneca, Roche, and Vaccitech and has patents in ChAdOx1 HBV and HCV vaccines.
Figures





References
-
- Swain S. L., McKinstry K. K., and Strutt T. M., “Expanding Roles for CD4+ T Cells in Immunity to Viruses,” Nature Reviews Immunology 12, no. 2 (2012): 136–148, https://www.nature.com/articles/nri3152. - PMC - PubMed
-
- Laidlaw B. J., Craft J. E., and Kaech S. M., “The Multifaceted Role of CD4+ T Cells in CD8+ T Cell Memory,” Nature Reviews Immunology 16, no. 2 (2016): 102–111, https://www.nature.com/articles/nri.2015.10. - PMC - PubMed
-
- Bennett S. R. M., Carbone F. R., Karamalis F., Flavell R. A., Miller J., and Heath W. R., “Help for Cytotoxic‐T‐Cell Responses Is Mediated by CD40 Signalling,” Nature 393, no. 6684 (1998): 478–480, https://www.nature.com/articles/30996. - PubMed
-
- Schoenberger S. P., Toes R. E. M., Van Dervoort E. I. H., Offringa R., and Melief C. J. M., “T‐Cell Help for Cytotoxic T Lymphocytes Is Mediated by CD40–CD40L Interactions,” Nature 393, no. 6684 (1998): 480–483, https://www.nature.com/articles/31002. - PubMed
-
- Castellino F., Huang A. Y., Altan‐Bonnet G., Stoll S., Scheinecker C., and Germain R. N., “Chemokines Enhance Immunity by Guiding Naive CD8+ T Cells to Sites of CD4+ T Cell–dendritic Cell Interaction,” Nature 440, no. 7086 (2006): 890–895, https://www.nature.com/articles/nature04651. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

