Safety, tolerability, pharmacokinetics, and pharmacodynamics of the oral allosteric TYK2 inhibitor ESK-001 using a randomized, double-blind, placebo-controlled study design
- PMID: 39604226
- PMCID: PMC11602527
- DOI: 10.1111/cts.70094
Safety, tolerability, pharmacokinetics, and pharmacodynamics of the oral allosteric TYK2 inhibitor ESK-001 using a randomized, double-blind, placebo-controlled study design
Abstract
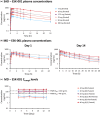
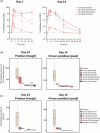
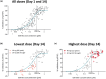
ESK-001 is a highly selective allosteric inhibitor of tyrosine kinase 2 (TYK2), which plays an essential role in mediating cytokine signaling in multiple immune-mediated diseases. In 2 phase I studies, a first-in-human single ascending dose (SAD) and multiple ascending dose (MAD) study and a multiple-dose (MD) study, we evaluated the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of orally administered ESK-001 in healthy participants using a randomized, double-blind, placebo-controlled study design. ESK-001 was rapidly absorbed with systemic exposures generally increasing dose-proportionally across all cohorts. The mean terminal half-life ranged from 8 to 13 h with no to minimal accumulation of ESK-001 following q.d. doses and ~2-fold accumulation following Q12 doses. Less than 1% of unchanged ESK-001 was eliminated in urine. ESK-001 inhibited the downstream TYK2 pathway as shown by inhibition of pSTAT1 expression. Transcriptomic analysis of unstimulated whole blood samples confirmed dose-dependent inhibition of Type I IFN-induced genes and SIGLEC1, a novel TYK2-responsive biomarker. By correlating PK exposure data with PD readouts, a strong PK/PD relationship was demonstrated. There were no deaths, serious treatment-emergent adverse events (TEAEs), nor severe TEAEs, and most TEAEs were mild in severity. In conclusion, ESK-001 was generally safe and well-tolerated in healthy participants, showed linear dose-dependent PK characteristics, and maximally inhibited TYK2-dependent pathways with a predictable concentration-dependent PK/PD relationship. These findings were used to select the dose range of ESK-001 for the STRIDE phase II trial in plaque psoriasis and to support further clinical development of ESK-001 in other TYK2-mediated diseases.
© 2024 The Author(s). Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
S.U., J.K.K, J.D.H., M.K.T., J.A.D., R.G.R., R.L., P.A.N., C.L.L. are employees of Alumis Inc. The authors have no other relationships or conflicts of interest to disclose.
Figures

References
-
- Zeng J, Luo S, Huang Y, Lu Q. Critical role of environmental factors in the pathogenesis of psoriasis. J Dermatol. 2017;44(8):863‐872. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

