Population genomics of Plasmodium ovale species in sub-Saharan Africa
- PMID: 39604397
- PMCID: PMC11603351
- DOI: 10.1038/s41467-024-54667-3
Population genomics of Plasmodium ovale species in sub-Saharan Africa
Abstract
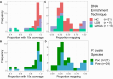
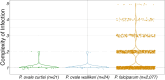
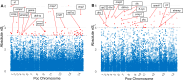
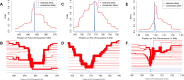
Plasmodium ovale curtisi (Poc) and Plasmodium ovale wallikeri (Pow) are relapsing malaria parasites endemic to Africa and Asia that were previously thought to represent a single species. Amid increasing detection of ovale malaria in sub-Saharan Africa, we present a population genomic study of both species across the continent. We conducted whole-genome sequencing of 25 isolates from Central and East Africa and analyzed them alongside 20 previously published African genomes. Isolates are predominantly monoclonal (43/45), with their genetic similarity aligning with geography. Pow shows lower average nucleotide diversity (1.8×10-4) across the genome compared to Poc (3.0×10-4) (p < 0.0001). Signatures of selective sweeps involving the dihydrofolate reductase gene have been found in both species, as are signs of balancing selection at the merozoite surface protein 1 gene. Differences in the nucleotide diversity of Poc and Pow may reflect unique demographic history, even as similar selective forces facilitate their resilience to malaria control interventions.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: J.B.P. reports research support from Gilead Sciences, non-financial support from Abbott Laboratories, and consulting for Zymeron Corporation, all outside the scope of this study. The remaining authors declare no competing interests. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Bill and Melinda Gates Foundation or other funders.
Figures


Update of
-
Population genomics of Plasmodium ovale species in sub-Saharan Africa.bioRxiv [Preprint]. 2024 Sep 19:2024.04.10.588912. doi: 10.1101/2024.04.10.588912. bioRxiv. 2024. Update in: Nat Commun. 2024 Nov 27;15(1):10297. doi: 10.1038/s41467-024-54667-3. PMID: 39345628 Free PMC article. Updated. Preprint.
References
-
- World Health Organization. World Malaria Report 2023 (World Health Organization, 2023).
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- R01 AI165537/AI/NIAID NIH HHS/United States
- R01 TW010870/TW/FIC NIH HHS/United States
- R01TW010870/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R21AI152260/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01 AI065537/AI/NIAID NIH HHS/United States
- F30 AI179111/AI/NIAID NIH HHS/United States
- R01 AI129812/AI/NIAID NIH HHS/United States
- R01AI129812/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R21AI148579/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01 AI132547/AI/NIAID NIH HHS/United States
- T32 AI070114/AI/NIAID NIH HHS/United States
- T32AI070114/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01AI65537/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R21 AI152260/AI/NIAID NIH HHS/United States
- R21 AI148579/AI/NIAID NIH HHS/United States
- R01AI132547/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01 AI137395/AI/NIAID NIH HHS/United States
- K24 AI134990/AI/NIAID NIH HHS/United States
- K24AI134990/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
LinkOut - more resources
Full Text Sources
Medical
Research Materials

