Neonatal immunity associated with heterologous HIV-1 neutralizing antibody induction in SHIV-infected Rhesus Macaques
- PMID: 39604409
- PMCID: PMC11603298
- DOI: 10.1038/s41467-024-54753-6
Neonatal immunity associated with heterologous HIV-1 neutralizing antibody induction in SHIV-infected Rhesus Macaques
Abstract
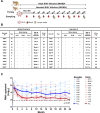
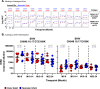
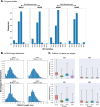
The details of the pediatric immune system that supports induction of antibodies capable of neutralizing geographically-diverse or heterologous HIV-1 is currently unclear. Here we explore the pediatric immune environment in neonatal macaque undergoing Simian-HIV infection. Simian-HIV infection of 11 pairs of therapy-naive dams and infant rhesus macaques for 24 months results in heterologous HIV-1 neutralizing antibodies in 64% of young macaques compared to 18% of adult macaques. Heterologous HIV-1 neutralizing antibodies emerge by 12 months post-infection in young macaques, in association with lower expression of immunosuppressive genes, fewer germinal center CD4 + T regulatory cells, and a lower ratio of CD4 + T follicular regulatory to helper cells. Antibodies from peripheral blood B cells in two young macaques following SHIV infection neutralize 13% of 119 heterologous HIV-1 strains and map to regions of canonical broadly neutralizing antibody epitopes on the envelope surface protein. Here we show that pediatric immunity to SHIV infection in a macaque model may inform vaccine strategies to induce effective HIV-1 neutralizing antibodies in infants and children prior to viral exposure.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- HHSN272201800004C/AI/NIAID NIH HHS/United States
- AI140897/Division of Intramural Research, National Institute of Allergy and Infectious Diseases (Division of Intramural Research of the NIAID)
- R37 AI150590/AI/NIAID NIH HHS/United States
- AI160607/Division of Intramural Research, National Institute of Allergy and Infectious Diseases (Division of Intramural Research of the NIAID)
- R01 AI160607/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

