Preclinical evaluation of polymer encapsulated carbon-based nano and microparticles for sentinel lymph node tattooing
- PMID: 39604460
- PMCID: PMC11603039
- DOI: 10.1038/s41598-024-80931-z
Preclinical evaluation of polymer encapsulated carbon-based nano and microparticles for sentinel lymph node tattooing
Abstract
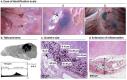
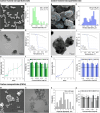
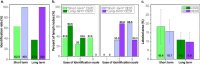
Selective sentinel lymph node biopsy (SNLB) is the standard method for detecting regional metastases in breast cancer patients. Identifying affected axillary lymph nodes before neoadjuvant treatment is crucial, as such treatment may alter drainage pathways and lymph node morphology, hindering the identification of sentinel lymph nodes. The use of carbon-based tattooing on sentinel lymph nodes (SLN) has been employed as a permanent tattooing method in clinical studies of Targeted Axillary Dissection (TAD), aiding in the SLN identification during surgery. Our study introduces a new method of lymph node tattooing based on poly lactic-co-glycolic (PLGA) particles with encapsulated carbon. This strategy substantially improves tattooing efficiency over single carbon suspensions currently used in clinical studies. We synthesized and characterized carbon-loaded PLGA micro- and nanoparticles, experimentally assessing their biological impact on porcine lymph nodes. The effect of particles' size and concentration was evaluated over time (from 1 to 16 weeks). Light and electron microscopy studies were conducted to characterize the cellular effects induced by the presence of these particles. Our findings reveal that the diverse physicochemical parameters of the particles interact differently with the lymphatic tissue, influencing their biodistribution within the lymph nodes and the intensity of the inflammatory response.
Keywords: Breast cancer; Carbon; Carbon-tattooing; Nanoparticles; Sentinel lymph node biopsy; Targeted-axillary dissection; Ultrastructure.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This research was approved by the Animal Research Ethics Committee of the University of Zaragoza under reference PI09/20. Competing interests: The authors declare no competing interests.
Figures







References
-
- Boileau, J. et al. Sentinel Node Biopsy after Neoadyuvant Chemotherapy in Biopsy-Proven node-positive breast Cancer: the SN FNAC Study. J. Clin. Oncol.20, 2588–2564 (2015). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

