Mechanical forces inducing oxaliplatin resistance in pancreatic cancer can be targeted by autophagy inhibition
- PMID: 39604540
- PMCID: PMC11603328
- DOI: 10.1038/s42003-024-07268-1
Mechanical forces inducing oxaliplatin resistance in pancreatic cancer can be targeted by autophagy inhibition
Abstract
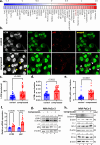
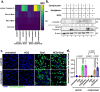
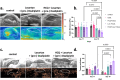
Pancreatic cancer remains one of the most lethal malignancies, with limited treatment options and poor prognosis. A common characteristic among pancreatic cancer patients is the biomechanically altered tumor microenvironment (TME), which among others is responsible for the elevated mechanical stresses in the tumor interior. Although significant research has elucidated the effect of mechanical stress on cancer cell proliferation and migration, it has not yet been investigated how it could affect cancer cell drug sensitivity. Here, we demonstrated that mechanical stress triggers autophagy activation, correlated with increased resistance to oxaliplatin treatment in pancreatic cancer cells. Our results demonstrate that inhibition of autophagy using hydroxychloroquine (HCQ) enhanced the oxaliplatin-induced apoptotic cell death in pancreatic cancer cells exposed to mechanical stress. The combined treatment of HCQ with losartan, a known modulator of mechanical abnormalities in tumors, synergistically enhanced the therapeutic efficacy of oxaliplatin in murine pancreatic tumor models. Furthermore, our study revealed that the use of HCQ enhanced the efficacy of losartan to alleviate mechanical stress levels and restore blood vessel integrity beyond its role in autophagy modulation. These findings underscore the potential of co-targeting mechanical stresses and autophagy as a promising therapeutic strategy to overcome drug resistance and increase chemotherapy efficacy.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
- 101069207/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- 101100769/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
LinkOut - more resources
Full Text Sources
Medical

