Late-life protein or isoleucine restriction impacts physiological and molecular signatures of aging
- PMID: 39604703
- PMCID: PMC11672203
- DOI: 10.1038/s43587-024-00744-7
Late-life protein or isoleucine restriction impacts physiological and molecular signatures of aging
Abstract
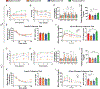
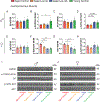
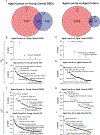
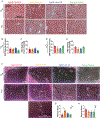
Restricting the intake of protein or the branched-chain amino acid isoleucine promotes healthspan and extends lifespan in young or adult mice. However, their effects when initiated in aged animals are unknown. Here we investigate the consequences of consuming a diet with 67% reduction of all amino acids (low AA) or of isoleucine alone (low Ile), in male and female C57BL/6J.Nia mice starting at 20 months of age. Both dietary regimens effectively promote overall metabolic health without reducing calorie intake. Both low AA and low Ile diets improve aspects of frailty and slow multiple molecular indicators of aging rate; however, the low Ile diet reduces grip strength in both sexes and has mixed, sexually dimorphic effects on the heart. These results demonstrate that low AA and low Ile diets can promote aspects of healthy aging in aged mice and suggest that similar interventions might promote healthy aging in older adults.
© 2024. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests: D.W.L. has received funding from, and is a scientific advisory board member of, Aeovian Pharmaceuticals, which seeks to develop novel, selective mTOR inhibitors for the treatment of various diseases. The other authors declare no competing interests.
Figures








Update of
-
Late-life isoleucine restriction promotes physiological and molecular signatures of healthy aging.bioRxiv [Preprint]. 2024 Jan 9:2023.02.06.527311. doi: 10.1101/2023.02.06.527311. bioRxiv. 2024. Update in: Nat Aging. 2024 Dec;4(12):1760-1771. doi: 10.1038/s43587-024-00744-7. PMID: 36798157 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
- R56 AG056771/AG/NIA NIH HHS/United States
- F31 AG066311/AG/NIA NIH HHS/United States
- I01 BX004031/BX/BLRD VA/United States
- AG082504/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- R01 AG062328/AG/NIA NIH HHS/United States
- F32 AG077916/AG/NIA NIH HHS/United States
- T32 AG000213/AG/NIA NIH HHS/United States
- T32AG000213, AG077916, AG084921/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- R01 DK125859/DK/NIDDK NIH HHS/United States
- K99 AG084921/AG/NIA NIH HHS/United States
- U01 AG081482/AG/NIA NIH HHS/United States
- F31 AG081115/AG/NIA NIH HHS/United States
- R01 DK133479/DK/NIDDK NIH HHS/United States
- IS1 BX005524/BX/BLRD VA/United States
- R01 AG056771/AG/NIA NIH HHS/United States
- AG081115/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- P30 CA014520/CA/NCI NIH HHS/United States
- F31 AG082504/AG/NIA NIH HHS/United States
- AG083290/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- U01 AG022303/AG/NIA NIH HHS/United States
- P30 AG024824/AG/NIA NIH HHS/United States
- R01 AG084156/AG/NIA NIH HHS/United States
- AG066311/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- F99 AG083290/AG/NIA NIH HHS/United States
- DK133479/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)
- RF1 AG056771/AG/NIA NIH HHS/United States
- R01 AG085898/AG/NIA NIH HHS/United States
- AG056771, AG062328, AG081482, AG084156, and AG085898/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

