Cell therapy for a rare disease- hairy cell leukemia variant
- PMID: 39604816
- PMCID: PMC11610546
- DOI: 10.1080/2162402X.2024.2432062
Cell therapy for a rare disease- hairy cell leukemia variant
Abstract
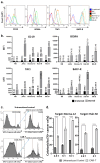
Hairy cell leukemia variant (HCL-v) is a rare malignancy of clonal mature B-cells that follows a chronic disease course. HCL-v patients are often resistant to purine nucleoside analogs, which are the first-line therapy. To address the shortcomings of current therapy for HCL-v, we investigated the activity of a BAFF ligand-based CAR-T cell which binds to all three BAFF receptors, BAFF-receptor, TACI, and BCMA. Here, we demonstrate that HCLv patient-derived cells highly express all three BAFF receptors and that BAFF CAR-T cells induce significant cytotoxicity in vitro against both cell lines and HCL-v patient cells. This cytotoxicity corresponds with significant CAR-T cell activation, degranulation, and release of pro-inflammatory cytokines after co-incubation with HCLv cells. Furthermore, we successfully generated BAFF CAR-T cells directly from an HCLv patient and observed direct autologous killing against patient tumor cells in vitro. These HCLv patient-derived CAR-T cells were also effective in killing the Hair-M cell line and tumor cells derived from a different HCLv patient. Lastly, we also developed two mouse xenograft models for HCL, a subcutaneous Bonna-12 model and intravenous Hair-M xenograft model. We observed decreases in tumor burden and prolonged overall survival without significant toxicity. In conclusion, here we show that BAFF CAR-T cells exert anti-tumor effects in vitro and in vivo against multiple cell lines and patient-derived HCL-v samples and may be a successful therapeutic strategy for HCLv patients.
Keywords: BAFF; CAR-T cells; HCL; cytotoxicity; leukemia.
Conflict of interest statement
RP serves as a Scientific Advisory Board Member of Luminary Therapeutics. BAFF CAR-T is licensed to Luminary Therapeutics.
Figures




References
-
- Epperla N, Pavilack M, Olufade T, Bashyal R, Li J, Kabadi SM, Yuce H, Andritsos L. Adverse event rates and economic burden associated with purine nucleoside analogs in patients with hairy cell leukemia: a US population-retrospective claims analysis. Orphanet J Rare Dis. 2020;15(1):47. doi:10.1186/s13023-020-1325-9. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
