Comparison of therapeutic effects of 0.05% Cyclosporine A versus 0.1% Fluorometholone in Chinese patients with mild dry eye unresponsive to artificial tears: a randomized control study
- PMID: 39604906
- PMCID: PMC11603962
- DOI: 10.1186/s12886-024-03771-5
Comparison of therapeutic effects of 0.05% Cyclosporine A versus 0.1% Fluorometholone in Chinese patients with mild dry eye unresponsive to artificial tears: a randomized control study
Abstract
Background: To assess and compare the therapeutic outcomes of 0.05% Cyclosporine A (CsA) ophthalmic solution versus 0.1% Fluorometholone (FML) eyedrops in Chinese patients with mild dry eye disease (DED) unresponsive to conventional artificial tears (AT).
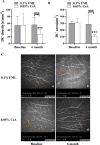
Methods: A total of 43 patients with mild DED, who have failed to respond to conventional AT therapy for over 3 months, were randomly assigned to receive either 0.05% CsA or 0.1% FML twice daily for 6-months. In addition, all the patients were instructed to use 0.1% SH 4 times a day as supplementary therapy. Dry eye examination, including Ocular Surface Disease Index (OSDI) questionnaire, non-invasive tear break-up time (NIBUT), Schirmer scores, corneal fluorescein staining (CFS) scores, and conjunctival goblet cell (CGC) density, intraocular pressure (IOP), Best corrected visual acuity (BCVA) was conducted at baseline and then evaluated at 1, 3, and 6 months after treatment. Corneal endothelial cell density, corneal dendritic cells (DCs) and nerves were assessed by in vivo confocal microscopy at baseline and 6 months after treatment.
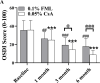
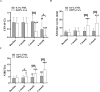
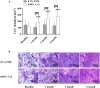
Results: At 3 and 6 months after treatment, OSDI scores in the 0.05% CsA group showed more improvement than those in the 0.1% FML group. CFS was significantly lower and Schirmer scores were significantly higher in 0.05% CsA group compared with 0.1% FML group. NIBUT improved significantly in both groups, with greater improvement in the 0.05% CsA group at the 1-, 3-, and 6-month visits. Throughout the duration of the study, the 0.1% FML group exhibited no notable enhancement in CGC density. Conversely, a substantial elevation in CGC density was observed in the 0.05% CsA group. After 6 months of treatment, significantly reduced corneal DC density and area were obtained in 0.05% CsA group as compared to 0.1% FML group, while there were no significant changes in cornea nerve fiber density, cornea nerve fiber length and cornea nerve fiber width in both groups. Additionally, after 6 months of treatment, neither group showed any statistically significant changes in IOP, BCVA or in corneal endothelial cell density.
Conclusion: The administration of 0.05% CsA proved effective in managing mild DED, offering a supplementary advantage in improving Schirmer scores, restoring CGC density and reducing corneal DC density compared to 0.1% FML eyedrops. Consequently, 0.05% CsA eyedrops are recommended as a safe and efficacious therapeutic alternative for patients with mild DED who fail to respond to conventional tear substitutes therapy.
Clinical trial registration number: Chinese Clinical Trial Registry, ChiCTR2200066441, Registered 06 December 2022-Retrospectively registered.
Keywords: 0.05% cyclosporine A; 0.1% fluorometholone; Conjunctival goblet cells; Corneal nerve; Dendritic cells; Mild dry eye disease.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The research was approved by the Ethics Committee of Tianjin Medical University Eye Hospital (ethics approval number: 2021KY-17). Written informed consent was obtained from all enrolled participants. Consent for publication: Not Applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Topical cyclosporine A therapy for dry eye syndrome.Cochrane Database Syst Rev. 2019 Sep 13;9(9):CD010051. doi: 10.1002/14651858.CD010051.pub2. Cochrane Database Syst Rev. 2019. PMID: 31517988 Free PMC article.
-
Topical fluorometholone treatment for ocular dryness in patients with Sjögren syndrome: a randomized clinical trial in China.Medicine (Baltimore). 2015 Feb;94(7):e551. doi: 10.1097/MD.0000000000000551. Medicine (Baltimore). 2015. PMID: 25700323 Free PMC article. Clinical Trial.
-
Impact of Topical 0.05% Cyclosporine A Eye Drops on Post-Femtosecond-Assisted Laser In Situ Keratomileusis Ocular Surface Recovery: A Randomized Clinical Trial.Eye Contact Lens. 2024 Aug 1;50(8):348-356. doi: 10.1097/ICL.0000000000001103. Epub 2024 Jun 11. Eye Contact Lens. 2024. PMID: 38865592 Free PMC article. Clinical Trial.
-
Corticosteroids Versus Cyclosporine for Subepithelial Infiltrates Secondary to Epidemic Keratoconjunctivitis: A Prospective Randomized Double-Blind Study.Cornea. 2021 Jun 1;40(6):726-732. doi: 10.1097/ICO.0000000000002589. Cornea. 2021. PMID: 33201059 Clinical Trial.
-
Safety and Efficacy of 0.1% Cyclosporine Solutions in Dry Eye Syndrome: A Systematic Review and Meta-Analysis of Randomized Clinical Trials.J Ocul Pharmacol Ther. 2025 May;41(4):199-209. doi: 10.1089/jop.2024.0169. Epub 2025 Feb 10. J Ocul Pharmacol Ther. 2025. PMID: 39929477
References
-
- Mohamed HB, Abd El-Hamid BN, Fathalla D, Fouad EA. Current trends in pharmaceutical treatment of dry eye disease: a review. Eur J Pharm Sciences: Official J Eur Federation Pharm Sci. 2022;175:106206. - PubMed
-
- Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, Na KS, Schaumberg D, Uchino M, Vehof J, et al. TFOS DEWS II Epidemiology Report. Ocul Surf. 2017;15(3):334–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

