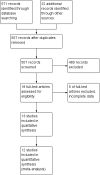
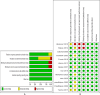
The use of misoprostol before hysteroscopy in Nulliparous women: a systematic review and meta-analysis of randomized controlled trials
- PMID: 39604937
- PMCID: PMC11600905
- DOI: 10.1186/s12884-024-06993-z
The use of misoprostol before hysteroscopy in Nulliparous women: a systematic review and meta-analysis of randomized controlled trials
Abstract
Objectives: To assess the value of misoprostol intake before hysteroscopy in nulliparous women.
Search strategy: Databases screening was done from inception to July 2023 using "Misoprostol" AND "Hysteroscopy" AND "Nullipara" and their MeSH terms as keywords.
Selection criteria: Thirteen studies were included in our analysis. Seven studies compared misoprostol to placebo, 3 studies compared it to dinoglandin, 1 study compared it to diclofenac and 4 studies compared different misoprostol doses and routes. These studies were conducted on 1528 participants,958 of them received misoprostol, 221 received dinoglandin, 51 received diclofenac and 308 received placebo.
Data collection and analysis: Extracted data included study place, participants number, inclusion and exclusion criteria, intervention details as dose, route, timing and comparotor, and hysteroscopy details.
Main results: Ease of cervical dilatation was reported in 3 studies (309 participants) and revealed an effect estimate mean difference (MD) of -0.57 [-1.72, 0.58] and a P value of 0.33. The time needed for cervical dilatation was reported in 6 studies (512 participants) and revealed a MD of -22.96 [-43.29, -2.62] and a P value of 0.03. The preoperative cervical width was reported in 4 studies (263 participants) and revealed MD of 1.69 [-0.09, 3.46] and a P value of 0.06. The number of women with failure of cervical dilatation or who needed further dilatation was reported in 4 studies (372 participants) and revealed a MD of 0.40 with [0.13, 1.17] 95% CI and a P value of 0.09. The preoperative pain was reported in 3 studies (351 participants) and revealed a MD of -0.56 [-2.30, 1.18] and a P value of 0.53. Total number of cases who experienced side effects and procedure complications were reported in 2 and 3 studies (249 and 252 participants) respectively and revealed an effect estimate Odd Ratio of 1.99 and 0.42 with [0.27, 14.67] and [0.14,1.32] 95% CI and a P value of 0.50 and 0.14 respectively. In the 3 studies comparing misoprostol to dinoglandin, The ease of cervical dilatation, time needed for cervical dilatation and preoperative cervical width were evaluated in 1,3 and 2 studies with 60, 436 and 376 participants respectively. The estimated MD were not estimated, 0.17 and 0.01; 95% CI were not estimated, [-4.70, 5.05], and [-0.78, 0.79]; P values of 0.94, 0.98 and 0.99 and I2 of 96%,95% and 74% respectively.
Conclusion: Misoprostol improved the time needed for cervical dilatation without affecting the rate of complications or drug side effects when compared to placebo but has similar outcomes to dinoglandin with higher side effects.
Registration number: CRD42023438432.
Keywords: Hysteroscopy; Misoprostol; Nullipara.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Comparison of vaginal misoprostol and dinoprostone for cervical ripening before diagnostic hysteroscopy in nulliparous women.Fertil Steril. 2015 May;103(5):1326-31. doi: 10.1016/j.fertnstert.2015.01.037. Epub 2015 Feb 21. Fertil Steril. 2015. PMID: 25712577 Clinical Trial.
-
Comparison of self-administered vaginal misoprostol versus placebo for cervical ripening prior to operative hysteroscopy using a sequential trial design.BJOG. 2008 Apr;115(5):663, e1-9. doi: 10.1111/j.1471-0528.2007.01628.x. Epub 2008 Jan 16. BJOG. 2008. PMID: 18201279 Free PMC article. Clinical Trial.
-
Efficacy and safety of oral vs vaginal misoprostol for cervical priming before hysteroscopy: A systematic review and meta-analysis.Eur J Obstet Gynecol Reprod Biol. 2019 Dec;243:111-119. doi: 10.1016/j.ejogrb.2019.10.023. Epub 2019 Oct 22. Eur J Obstet Gynecol Reprod Biol. 2019. PMID: 31689673
-
Sublingual misoprostol for cervical ripening before diagnostic hysteroscopy in premenopausal women: a randomized, double blind, placebo-controlled trial.Fertil Steril. 2010 May 1;93(7):2400-4. doi: 10.1016/j.fertnstert.2009.01.073. Epub 2009 Feb 24. Fertil Steril. 2010. PMID: 19243750 Clinical Trial.
-
[Cervical ripening using misoprostol before hysteroscopy].Gynecol Obstet Fertil. 2006 Jan;34(1):49-53. doi: 10.1016/j.gyobfe.2005.11.004. Epub 2006 Jan 18. Gynecol Obstet Fertil. 2006. PMID: 16413811 Review. French.
References
-
- Vitner D, Filmer S, Goldstein I, Khatib N, Weiner Z. A comparison between ultrasonography and hysteroscopy in the diagnosis of uterine pathology. Eur J Obstet Gynecol Reprod Biol. 2013;171:143–5. - PubMed
-
- Bettocchi S, Ceci O, Vicino M, Marello F, Impedovo L, Selvaggi L. Diagnostic inadequacy of dilatation and curettage. Fertil Steril. 2001;75:803–5. - PubMed
-
- van Dongen H, de Kroon CD, Jacobi CE, Trimbos JB, Jansen FW. Diagnostic hysteroscopy in abnormal uterine bleeding: a systematic review and meta-analysis. BJOG. 2007;114:664–75. - PubMed
-
- Morgan M, Dodds W, Wolfe C, Raju S. Women’s views and experiences of outpatient hysteroscopy: implications for a patient-centered service. Nurs Health Sci. 2004;6:315–20. - PubMed
-
- Gupta JK, Clark TJ, More S, Pattison H. Patient anxiety and experiences associated with an outpatient one-stop see and treat hysteroscopy clinic. Surg Endosc. 2004;18:1099–104. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources