A strain-guided trial of cardioprotection in early-stage breast cancer patients on anti-HER2 therapy (PROTECT HER2)
- PMID: 39605014
- PMCID: PMC11600554
- DOI: 10.1186/s40959-024-00291-5
A strain-guided trial of cardioprotection in early-stage breast cancer patients on anti-HER2 therapy (PROTECT HER2)
Abstract
Background: Global longitudinal strain (GLS) has been used to identify patients at risk for cancer-therapy related cardiac dysfunction (CTRCD). However, there is limited data on the effectiveness of initiating cardioprotective therapy based on a strain-guided strategy in early stage HER2+ breast cancer patients. This randomized clinical trial assessed if treatment with carvedilol based on a strain-guided strategy can prevent development of CTRCD in HER2+ breast cancer patients on non-anthracycline based regimens.
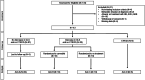
Methods: Study participants were prospectively assigned to one of four arms. Patients with normal LVEF and GLS remained in Arm A. Patients whose GLS decreased by > 15% from baseline or to < -15% during follow up were randomized 1:1 to prophylactic carvedilol (Arm B) or no therapy (Arm C). Patients who developed CTRCD were assigned to Arm D. The primary endpoint was GLS stability. The secondary endpoints were development of CTRCD and rate of anti-HER2 treatment interruption.
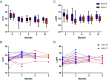
Results: Among 110 patients who completed follow up, 84 were assigned to Arm A, 10 each were randomized to Arms B or C, and 6 were assigned to Arm D. At the end of the study period, there were no significant differences in GLS stability, development of CTRCD, or number of cancer therapy cycles completed between patients who did and did not receive cardioprotective therapy.
Conclusions: In this prospective randomized GLS-guided study of prophylactic carvedilol in early stage HER2+ breast cancer patients on non-anthracycline regimens, there were no significant difference between groups in GLS stability, CTRCD or trastuzumab cycles held. These findings may identify a low-risk group of patients who may be considered for less intensive cardiac surveillance.
Trial registration: https://clinicaltrials.gov/study/NCT02993198 . Start date: 4/2015. This trial included patients who were retrospectively registered.
Keywords: CTRCD; Cardioprotective therapy; Global longitudinal strain; HER2+ breast cancer.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Northwestern University Institutional Review Board. Consent for publication: Not applicable. Competing interests: SR is a consultant for Abbott Laboratories. GM is a full-time employee and shareholder of Abbott Laboratories. NA received research funding from Abbott Laboratories. The other authors declare no conflict of interest.
Figures
References
-
- Gong I, Verma S, Yan AT. Long-term cardiovascular outcomes and overall survival of early-stage breast cancer patients with early discontinuation of trastuzumab: a population-based study. Breast Cancer Res Treat. 2016;157(3):535–44. - PubMed
-
- Thavendiranathan P, Poulin F, Lim K, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. 2014;63(25):2751–68. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
