Mortality in septic patients treated with short-acting betablockers: a comprehensive meta-analysis of randomized controlled trials
- PMID: 39605034
- PMCID: PMC11603935
- DOI: 10.1186/s13054-024-05174-w
Mortality in septic patients treated with short-acting betablockers: a comprehensive meta-analysis of randomized controlled trials
Abstract
Background: Treatment with short-acting betablockers in septic patients remains controversial. Two recent large multicenter trials have provided additional evidence on this therapeutic approach. We thus performed a meta-analysis, including the most recent data, to evaluate the potential impacts of treatment with short-acting betablockers on mortality in adult septic patients.
Methods: The data search included PubMed, Web of Science, ClinicalTrials.gov and the Cochrane Library. A meta-analysis of all eligible peer-reviewed studies was performed in accordance with the PRISMA statement. Only randomized, controlled studies with valid classifications of sepsis and intravenous treatment with short-acting betablockers (landiolol or esmolol) were included. Short-term mortality served as the primary endpoint. Secondary endpoints included effects on short-term mortality regarding patient age and cardiac rhythm.
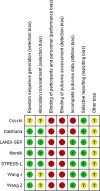
Results: A total of seven studies summarizing 854 patients fulfilled the predefined criteria and were included. Short-term mortality as well as pooled mortality (longest period of data on mortality) was not significantly impacted by treatment with short-acting betablockers when compared to the reference treatment (Risk difference, - 0.10 [95% CI, - 0.22 to 0.02]; p = 0.11; p for Cochran's Q test = 0.001; I2 = 73%). No difference was seen when comparing patients aged < 65 versus ≥ 65 years (p = 0.11) or sinus tachycardia with atrial fibrillation (p = 0.27). Despite statistical heterogeneity, no significant publication bias was observed.
Conclusion: Administration of short-acting betablockers did not reduce short-term mortality in septic patients with persistent tachycardia. Future studies should also provide extensive hemodynamic data to enable characterization of cardiac function before and during treatment.
Keywords: Betablocker; Esmolol; Landiolol; Mortality; Sepsis; Septic shock.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Human ethics and consent to participate: Not applicable. Consent for publication: All authors have read and approved the submission of this manuscript. Competing interests: M.A., P.N., S.K., R.B., and S.S.S. report no competing interests. S. R. served as a speaker for AOP Health. T.W. was the Chief Investigator for STRESS‐L which was funded by the National Institute for Health Research (NIHR) Efficacy and Mechanism Evaluation (Project Number: EME‐14/150/85) and during the conduct of the study, he received personal fees and non‐financial support from AOP Orphan, manufacturer of landiolol. M.S. (or Institution) receives funding for studies, advisory board or speaker fees from the NIHR, Medical Research Council, AOP Pharma, Aptarion, Biomerieux, Biotest, deePull, Deltex Medical, Gentian, Matisse, Roche Diagnostics, Safeguard Biosystems, Volition. Singer is Sepsis Topic Advisor for NICE and sits on the council of the International Sepsis Forum.
Figures


References
-
- Fleischmann C, Hartmann M, Hartog C, Welte T, Heublein S, Thomas-Rueddel D, et al. Epidemiology of sepsis in Germany: incidence, mortality and associated costs of care 2007–2013. Intensive Care Med Exp. 2015;3:A50. 10.1186/2197-425X-3-S1-A50.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

