Implementation and evaluation of a navigation program for people with cancer in old age and their family caregivers: study protocol for the EU NAVIGATE International Pragmatic Randomized Controlled Trial
- PMID: 39605055
- PMCID: PMC11603903
- DOI: 10.1186/s13063-024-08633-5
Implementation and evaluation of a navigation program for people with cancer in old age and their family caregivers: study protocol for the EU NAVIGATE International Pragmatic Randomized Controlled Trial
Abstract
Background: Cancer navigation programs aim to support, educate, and empower patients and families, addressing barriers to diagnostics, treatment, and care. Navigators engage with people to ensure timely access to services and resources. While promising for older people with cancer, these programs are scarce in Europe, and research on their effectiveness and implementation is limited. We describe the protocol of the EU NAVIGATE randomized controlled trial, aimed to evaluate (1) effectiveness and cost-effectiveness of NavCare-EU, an intervention that aims to support older people with cancer throughout their illness trajectory, spanning the continuum of supportive, palliative, and end-of-life care, and (2) the intervention's implementation processes and feasibility of its integration into different health care systems in Europe, contextual barriers and facilitators for effective and sustainable implementation, and mechanisms involved in reaching the outcomes.
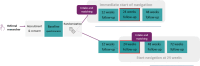
Methods: We will conduct a multisite pragmatic fast-track randomized controlled trial with embedded convergent mixed-method process evaluation in Belgium, Ireland, Italy, Netherlands, Poland, and Portugal. The study targets people with cancer and declining health, 70 years or older, and their close family caregivers. The trial compares the NavCare-EU intervention plus standard care with standard care alone. We will perform a baseline measurement prior to randomization and follow-up measurements at 12 weeks, 24 weeks, and 48 weeks in intervention and control group, and an additional measurement at 72 weeks in the control group. Primary outcomes, measured at 24 weeks are (1) the older person's global health status/quality of life, a 2-item subscale from EORTC-QLQ-C30 (revised) measuring health-related quality of life, (2) level of social support measured with Medical Outcomes Study Social Support Survey (MOS-SSS scale). The study will include at least 246 older persons with completed global health status/quality of life at 24 weeks.
Discussion: The EU NAVIGATE trial will cross-nationally test the effectiveness and cost-effectiveness of a navigation intervention for older people with cancer and their family caregivers, and its implementation in different health care systems in Europe. As continuity and access to health, social, and community care is a priority for patients and caregivers, the trial is timely and critically needed.
Trial registration: Clinicaltrials.gov: identifier NCT06110312 (2023/10/31).
Keywords: Cancer; Family caregivers; Navigation; Older persons; Palliative care; Public Health; Quality of life; Supportive care; Volunteers.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Ethics approval from the relevant ethics committees were obtained in all participating countries. Belgium: Commissie Medische Ethiek, 09/08/2023; Ireland: SJH/TUH Joint Research Ethics Committee, 14/11/2023; Italy: Comitato Etico Territoriale Lombardia 4, Istituto Tumori, 31/07/2023; the Netherlands: METC Amsterdam UMC, 22/08/2023; Portugal: Ethics Committee of the Faculty of Medicine of the University of Coimbra and Ethics Committee of the Portuguese Institute of Oncology of Coimbra Francisco Gentil, 25/09/2023; Poland: Komisja Bioetyczna, Uniwersytetu Jagiellońskiego, 14/06/2023. If all eligibility criteria are met, the researcher or research assistant will obtain informed consent from both the older person and the close family caregiver (if there is one). Patients and caregivers will be given the time to consider participation and will be assured that they are free to withdraw their participation without any effect on their care. Written consent will be obtained without any coercion of study participants. The research team will provide all participants with full disclosure about the nature and goal of the study. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures
References
-
- Hub. ES. 2020 Cancer incidence and mortality in EU-27 countries. 2020 [Available from: https://joint-research-centre.ec.europa.eu/jrc-news-and-updates/2020-can....
-
- Cancer. WIAfRo. Global cancer burden growing, amidst mounting need for services. 2024 [Available from: https://www.iarc.who.int/news-events/global-cancer-burden-growing-amidst... - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials