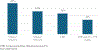Upadacitinib for Induction of Remission in Pediatric Ulcerative Colitis: An International Multicenter Study
- PMID: 39605286
- PMCID: PMC12548835
- DOI: 10.1093/ecco-jcc/jjae182
Upadacitinib for Induction of Remission in Pediatric Ulcerative Colitis: An International Multicenter Study
Abstract
Background and aims: Data on upadacitinib therapy in children with ulcerative colitis (UC) or unclassified inflammatory bowel disease (IBD-U) are scarce. We aimed to evaluate the effectiveness and safety of upadacitinib as an induction therapy in pediatric UC or IBD-U.
Methods: In this multicenter retrospective study, children treated with upadacitinib for induction of remission of active UC or IBD-U from 30 centers worldwide were enrolled. Demographic, clinical, and laboratory data, as well as adverse events (AEs), were recorded at Week 8 post-induction.
Results: One hundred children were included (90 UC and 10 IBD-U, median age 15.6 [interquartile range 13.3-17.1] years). Ninety-eight were previously treated with biologic therapies, and 76 were treated with ≥2 biologics. At the end of the 8-week induction period, clinical response, clinical remission, and corticosteroid-free clinical remission (CFR) were observed in 84%, 62%, and 56% of the children, respectively. Normal C-reactive protein and fecal calprotectin (FC) <150 mcg/g were achieved in 75% and 50%, respectively. Combined CFR and FC remission was observed in 18/46 (39%) children with available data at 8 weeks. Adverse events were recorded in 37 children, including 1 serious AE of an appendiceal neuroendocrine tumor. The most frequent AEs were hyperlipidemia (n = 13), acne (n = 12), and infections (n = 10, 5 of whom with herpes viruses).
Conclusions: Upadacitinib is an effective induction therapy for refractory pediatric UC and IBD-U. Efficacy should be weighed against the potential risks of AEs.
Keywords: Inflammatory bowel disease; JAK inhibitors; children.
© The Author(s) 2024. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
Conflict of Interest
These authors disclose the following:
D.T.: Last 3 years, D.T. received consultation fee, research grant, royalties, or honorarium from Janssen, Pfizer, Shaare Zedek Medical Center, Hospital for Sick Children, Ferring, Abbvie, Takeda, Prometheus Biosciences, Lilly, SorrisoPharma, Boehringer Ingelheim, Galapagos, BMS, AlfaSigma MTD received consultant fees from Pfizer and BMS. M.G. is a Member of the CICRA Advisory Board (Crohn’s in Childhood Research Association). M.G. is currently involved in pharmaceutical trials sponsored by Abbvie. D.S.S. received lecturing fees from Takeda. M.B. received a consultant fee from Pfizer. O.H. received lectures/congress fees/consultancy from MSD, Abbvie, Takeda, Nutricia, Sandoz, Lilly. D.L.S.: Consultant: Pharming Pharm, Nestle. For the past 3 years, B.K. has received speaker fee, consultation fee, or research grant from Celltrion, Janssen, Abbvie, Takeda, Yuhan, Yungjin, JW Pharmaceutical, and Samsung Bioepis. H.H.U. has received research support or consultancy fees from J&J, Eli Lilly, Bristol Myers Squibb and AbbVie. HHU is supported by the National Institute for Health Research, Oxford Biomedical Research Centre, University of Oxford (HHU), and by The Leona M. and Harry B. Helmsley Charitable Trust. L.H.: received speaking fee from Abbvie. G.D.: Lectures/congress fees/consultancy: Nestlé Health Science and Pfizer.
Figures
References
-
- Turner D, Ruemmele FM, Orlanski-Meyer E, et al. Management of paediatric ulcerative colitis, part 1: ambulatory care-an evidence-based guideline from European Crohn’s and Colitis Organization and European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 2018;67:257–91. - PubMed
-
- Atia O, Shavit-Brunschwig Z, Mould DR, et al. Outcomes, dosing, and predictors of vedolizumab treatment in children with inflammatory bowel disease (VEDOKIDS): a prospective, multicentre cohort study. Lancet Gastroenterol Hepatol 2023;8:31–42. - PubMed
-
- Voss J, Graff C, Schwartz A, et al. Pharmacodynamics of a novel Jak1 selective inhibitor in rat arthritis and anemia models and in healthy human subjects. Ann Rheum Dis 2014;73:222.2–222.
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials