The anticholinergic burden in patients with chronic kidney disease: Patterns, risk factors, and the link with cognitive impairment
- PMID: 39605299
- PMCID: PMC11825999
- DOI: 10.1111/jgs.19283
The anticholinergic burden in patients with chronic kidney disease: Patterns, risk factors, and the link with cognitive impairment
Abstract
Background: People with chronic kidney disease (CKD) have an elevated risk of cognitive impairment (CI). Medications with anticholinergic activity are recognized for their adverse reactions on central nervous system. The putative association between the anticholinergic burden and CI has not previously been evaluated in patients with CKD. The study aimed to (i) describe prescriptions of medications with anticholinergic activity, (ii) analyze factors associated with these prescriptions, and (iii) evaluate the anticholinergic burden's association with cognitive performance.
Methods: CKD-REIN, a prospective cohort study, enrolled nephrology outpatients with a confirmed diagnosis of CKD (eGFR <60 mL/min/1.73m2). Drug prescriptions were recorded prospectively during the 5-year follow-up. Mini Mental State Examination (MMSE) was assessed at baseline and CI was defined as an MMSE score <24/30. For each patient, the anticholinergic burden was determined by summing the Anticholinergic Cognitive Burden (ACB) scores of all prescription drugs at baseline. Multinomial logistic regression was used to analyze factors associated with the ACB score. Logistic regression was used to evaluate the association between the cognitive impairment and the anticholinergic burden at baseline.
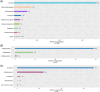
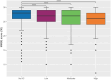
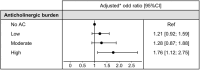
Results: At baseline, 3007 patients (median age [IQR], 69[60-76]; 65% men) had MMSE data and were included. 1549 (52%) of these patients were taking at least one drug with anticholinergic properties. Most (1092; 70%) had a low anticholinergic burden, 294 (19%) had a moderate anticholinergic burden, and 163 (11%) had a high anticholinergic burden. A history of neurological/psychiatric disorders and a higher number of daily drugs were associated with a greater probability of having a high anticholinergic burden (odds ratio (OR) [95% confidence interval (95% CI)] = 1.88[1.29;2.74] and 1.53[1.45;1.61], respectively). Patients with a high anticholinergic burden had a significantly higher probability of presenting cognitive impairment, compared with patients without an anticholinergic burden (OR[95% CI] = 1.76[1.12;2.75]) after adjustment for sociodemographic factors, comorbidities, laboratory data, and the number of medications taken daily.
Conclusions: The results of our study emphasize the need for caution in the prescription of drugs with anticholinergic properties to patients with CKD.
Keywords: anticholinergic; chronic kidney disease; cognition; drug use; pharmacoepidemiology.
© 2024 The Author(s). Journal of the American Geriatrics Society published by Wiley Periodicals LLC on behalf of The American Geriatrics Society.
Conflict of interest statement
A.M., H.L., S.M.L., S.L., M.P., M.L., and C.J. have nothing to declare. Z.A.M. reports having received grants for CKD‐REIN and other research projects from Amgen, Baxter, Fresenius Medical Care, GlaxoSmithKline, Merck Sharp and Dohme‐Chibret, Sanofi‐ Genzyme, Lilly, Otsuka, AstraZeneca, Vifor, and the French government, as well as fees and grants to charities from AstraZeneca, Boehringer Ingelheim, and GlaxoSmithKline. N.A.P. declares financial support from pharmaceutical companies who are members of the CKD‐REIN public‐private partnership: Fresenius Medical Care, GlaxoSmithKline, Vifor France, and Boehringer Ingelheim; all grants were made to Paris Saclay University.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

