This is a preprint.
Unbiased discovery of antibody therapies that stimulate macrophage-mediated destruction of B-cell lymphoma
- PMID: 39605364
- PMCID: PMC11601295
- DOI: 10.1101/2024.11.13.623229
Unbiased discovery of antibody therapies that stimulate macrophage-mediated destruction of B-cell lymphoma
Abstract
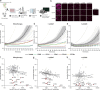
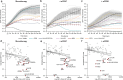
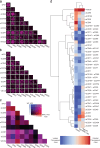
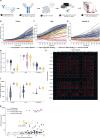
Macrophages are critical effectors of antibody therapies for lymphoma, but the best targets for this purpose remain unknown. Here, we sought to define a comprehensive repertoire of cell surface antigens that can be targeted to stimulate macrophage-mediated destruction of B-cell lymphoma. We developed a high-throughput assay to screen hundreds of antibodies for their ability to provoke macrophages to attack B-cell lymphoma cells. Across both mouse and human systems, we identified multiple unappreciated targets of opsonization as well as putative immune checkpoints. We used this information to engineer a compendium of 156 bispecific antibodies, and we identified dozens of bispecifics that dramatically stimulate macrophage-mediated cytotoxicity of lymphoma cells. Among these, a bispecific comprising a SIRPα decoy domain and a CD38-targeting arm (WTa2d1×CD38) exhibited maximal efficacy while minimizing the risk of hematologic toxicity. This bispecific stimulated robust anti-tumor responses in multiple xenograft models of aggressive B-cell lymphoma. Our approach can be directly applied to other cancers to rapidly discover bispecific antibodies that leverage anti-tumor responses by macrophages or other innate immune cells.
Conflict of interest statement
Competing interests JR, CPG, KV, AM, and KW have filed US patent applications related to this work. KV is a former employee and equity owner of DEM Biopharma. MC has received research funding from Janssen, Genentech and AstraZeneca. KW reports patents/royalties (Stanford University, Whitehead Institute, Forty Seven, Gilead Sciences, ALX Oncology, DEM Biopharma); co-founder, scientific advisory board member, and equity holder (ALX Oncology, DEM Biopharma, Solu Therapeutics), stock ownership (Ginkgo Bioworks). The other authors declare no competing interests.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
