This is a preprint.
Arginyltransferase1 drives a mitochondria-dependent program to induce cell death
- PMID: 39605427
- PMCID: PMC11601567
- DOI: 10.1101/2024.11.22.624728
Arginyltransferase1 drives a mitochondria-dependent program to induce cell death
Update in
-
Arginyltransferase1 drives a mitochondria-dependent program to induce cell death.Cell Death Dis. 2025 Aug 16;16(1):622. doi: 10.1038/s41419-025-07917-1. Cell Death Dis. 2025. PMID: 40818972 Free PMC article.
Abstract
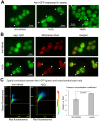
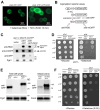
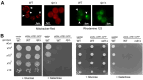
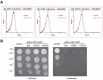
Cell death regulation is essential for stress adaptation and/or signal response. Past studies have shown that eukaryotic cell death is mediated by an evolutionarily conserved enzyme, arginyltransferase1 (Ate1). The downregulation of Ate1, as seen in many types of cancer, prominently increases cellular tolerance to a variety of stressing conditions. Conversely, in yeast and mammalian cells, Ate1 is elevated under acute oxidative stress conditions and this change appears to be essential for triggering cell death. However, studies of Ate1 were conventionally focused on its function in inducing protein degradation via the N-end rule pathway in the cytosol, leading to an incomplete understanding of the role of Ate1 in cell death. Our recent investigation shows that Ate1 dually exists in the cytosol and mitochondria, the latter of which has an established role in cell death initiation. Here, by using budding yeast as a model organism, we found that mitochondrial translocation of Ate1 is promoted by the presence of oxidative stressors and is essential for inducing cell death with characteristics of apoptosis. Also, we found that Ate1-induced cell death is dependent on the formation of the mitochondrial permeability pore and at least partly dependent on the action of mitochondria-contained factors including the apoptosis-inducing factor, but is not directly dependent on mitochondrial electron transport chain activity or its derived reactive oxygen species (ROS). Furthermore, our evidence suggests that, contrary to widespread assumptions, the cytosolic protein degradation pathways including ubiquitin-proteasome, autophagy, or endoplasmic reticulum (ER) stress response has little or negligible impacts on Ate1-induced cell death. We conclude that Ate1 controls the mitochondria-dependent cell death pathway.
Keywords: Arginylation; Ate1; apoptosis; arginyltransferase; mitochondrial; posttranslational modification; programmed cell death; ubiquitination.
Figures



References
-
- Kaji H., Novelli G.D., and Kaji A., A Soluble Amino Acid-Incorporating System from Rat Liver. Biochim Biophys Acta, 1963. 76: p. 474–7. - PubMed
-
- Bachmair A., Finley D., and Varshavsky A., In vivo half-life of a protein is a function of its amino-terminal residue. Science, 1986. 234(4773): p. 179–86. - PubMed
-
- Chakraborty G., et al. , Posttranslational protein modification by amino acid addition in regenerating optic nerves of goldfish. J Neurochem, 1986. 46(3): p. 726–32. - PubMed
-
- Shyne-Athwal S., et al. , Protein modification by amino acid addition is increased in crushed sciatic but not optic nerves. Science, 1986. 231(4738): p. 603–5. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
