This is a preprint.
Adaptations in gut Bacteroidales facilitate stable co-existence with their lytic bacteriophages
- PMID: 39605433
- PMCID: PMC11601342
- DOI: 10.1101/2024.11.17.624012
Adaptations in gut Bacteroidales facilitate stable co-existence with their lytic bacteriophages
Update in
-
Adaptations in gut Bacteroidales facilitate stable co-existence with their lytic bacteriophages.Gut Microbes. 2025 Dec;17(1):2507775. doi: 10.1080/19490976.2025.2507775. Epub 2025 May 23. Gut Microbes. 2025. PMID: 40407098 Free PMC article.
Abstract
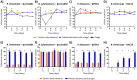
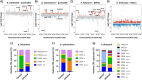
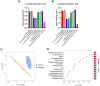
Background: Bacteriophages (phages) and bacteria within the gut microbiome persist in long-term stable coexistence. These interactions are driven by eco-evolutionary dynamics, where bacteria employ a variety of mechanisms to evade phage infection, while phages rely on counterstrategies to overcome these defences. Among the most abundant phages in the gut are the crAss-like phages that infect members of the Bacteroidales, in particular Bacteroides. In this study, we explored some of the mechanisms enabling the co-existence of four phage-Bacteroidales host pairs in vitro using a multi-omics approach (transcriptomics, proteomics and metabolomics). These included three Bacteroides species paired with three crAss-like phages (Bacteroides intestinalis and ϕcrAss001, Bacteroides xylanisolvens and ϕcrAss002, and an acapsular mutant of Bacteroides thetaiotaomicron with DAC15), and Parabacteroides distasonis paired with the siphovirus ϕPDS1.
Results: We show that phase variation of individual capsular polysaccharides (CPSs) is the primary mechanism promoting phage co-existence in Bacteroidales, but this is not the only strategy. Alternative resistance mechanisms, while potentially less efficient than CPS phase variation, can be activated to support bacterial survival by regulating gene expression and resulting in metabolic adaptations, particularly in amino acid degradation pathways. These mechanisms, also likely regulated by phase variation, enable bacterial populations to persist in the presence of phages, and vice versa. An acapsular variant of B. thetaiotaomicron demonstrated broader transcriptomic, proteomic, and metabolomic changes, supporting the involvement of additional resistance mechanisms beyond CPS variation.
Conclusions: This study advances our understanding of long-term phage-host interaction, offering insights into the long-term persistence of crAss-like phages and extending these observations to other phages, such as ϕPDS1. Knowledge of the complexities of phage-bacteria interactions is essential for designing effective phage therapies and improving human health through targeted microbiome interventions.
Keywords: Bacteriophages; Bacteroides; Crassvirales; Parabacteroides; co-culture; crAssphages; gut microbiome; intestinal microbiota; phage-bacteria interaction; virome.
Figures

References
-
- Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19(1):55–71. - PubMed
-
- Shareefdeen H, Hill C. The gut virome in health and disease: new insights and associations. Curr Opin Gastroenterol. 2022;38(6):549–554. - PubMed
-
- Tobin CA, Hill C, Shkoporov AN. Factors Affecting Variation of the Human Gut Phageome. Annu Rev Microbiol. 2023;77:363–379. - PubMed
-
- Shkoporov AN, Turkington CJ, Hill C. Mutualistic interplay between bacteriophages and bacteria in the human gut. Nat Rev Microbiol. 2022;20(12):737–749. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
