This is a preprint.
Impaired complement regulation drives chronic lung allograft dysfunction after lung transplantation
- PMID: 39605452
- PMCID: PMC11601477
- DOI: 10.1101/2024.11.17.623951
Impaired complement regulation drives chronic lung allograft dysfunction after lung transplantation
Update in
-
Impaired complement regulation drives chronic lung allograft dysfunction after lung transplantation.J Clin Invest. 2025 Nov 11:e188891. doi: 10.1172/JCI188891. Online ahead of print. J Clin Invest. 2025. PMID: 41217841
Abstract
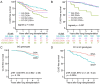
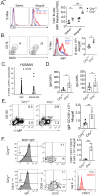
A greater understanding of chronic lung allograft dysfunction (CLAD) pathobiology, the primary cause of mortality after lung transplantation, is needed to improve outcomes. The complement system links innate to adaptive immune responses and is activated early post-lung transplantation to form the C3 convertase, a critical enzyme that cleaves the central complement component C3. We hypothesized that LTx recipients with a genetic predisposition to enhanced complement activation have worse CLAD-free survival mediated through increased adaptive alloimmunity. We interrogated a known functional C3 polymorphism (C3R102G) that increases complement activation through impaired C3 convertase inactivation in two independent LTx recipient cohorts. C3R102G, identified in at least one out of three LTx recipients, was associated with worse CLAD-free survival, particularly in the subset of recipients who developed donor-specific antibodies (DSA). In a mouse orthotopic lung transplantation model, impaired recipient complement regulation resulted in more severe obstructive airway lesions when compared to wildtype controls, despite only moderate differences in graft-infiltrating effector T cells. Impaired complement regulation promoted the intragraft accumulation of memory B cells and antibody-secreting cells, resulting in increased DSA levels. In summary, genetic predisposition to complement activation is associated with B cell activation and worse CLAD-free survival.
Conflict of interest statement
Conflict of interest statement: The authors have declared that no conflict of interest exists.
Figures


References
-
- DerHovanessian A, et al. Chronic Lung Allograft Dysfunction: Evolving Concepts and Therapies. Semin Respir Crit Care Med. 2018;39(2):155–171. - PubMed
-
- Venado A, Kukreja J, Greenland JR. Chronic Lung Allograft Dysfunction. Thorac Surg Clin. 2022;32(2):231–242. - PubMed
-
- Kulkarni HS, et al. Bronchiolitis-Obliterans Syndrome-Free Survival of Lung Transplant Recipients - Analysis of the ISHLT Registry. The Journal of Heart and Lung Transplantation. 2017;36(4):S310–S311.
-
- Vandermeulen E, et al. Humoral immunity in phenotypes of chronic lung allograft dysfunction: A broncho-alveolar lavage fluid analysis. Transpl Immunol. 2016;38:27–32. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
