This is a preprint.
Interspecies Organoids Reveal Human-Specific Molecular Features of Dopaminergic Neuron Development and Vulnerability
- PMID: 39605599
- PMCID: PMC11601475
- DOI: 10.1101/2024.11.14.623592
Interspecies Organoids Reveal Human-Specific Molecular Features of Dopaminergic Neuron Development and Vulnerability
Abstract
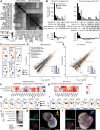
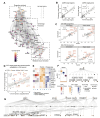
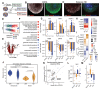
The disproportionate expansion of telencephalic structures during human evolution involved tradeoffs that imposed greater connectivity and metabolic demands on midbrain dopaminergic neurons. Despite the central role of dopaminergic neurons in human-enriched disorders, molecular specializations associated with human-specific features and vulnerabilities of the dopaminergic system remain unexplored. Here, we establish a phylogeny-in-a-dish approach to examine gene regulatory evolution by differentiating pools of human, chimpanzee, orangutan, and macaque pluripotent stem cells into ventral midbrain organoids capable of forming long-range projections, spontaneous activity, and dopamine release. We identify human-specific gene expression changes related to axonal transport of mitochondria and reactive oxygen species buffering and candidate cis- and trans-regulatory mechanisms underlying gene expression divergence. Our findings are consistent with a model of evolved neuroprotection in response to tradeoffs related to brain expansion and could contribute to the discovery of therapeutic targets and strategies for treating disorders involving the dopaminergic system.
Keywords: Brain evolution; Parkinson’s disease; dopaminergic; iPSCs; midbrain; organoids; oxidative stress; single cell genomics.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
-
- Brisch R., Saniotis A., Wolf R., Bielau H., Bernstein H.-G., Steiner J., Bogerts B., Braun K., Jankowski Z., Kumaratilake J., et al. (2014). The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: old fashioned, but still in vogue. Front. Psychiatry 5, 47. 10.3389/fpsyt.2014.00047. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
