This is a preprint.
Mechanisms of growth-dependent regulation of the Gin4 kinase
- PMID: 39605684
- PMCID: PMC11601526
- DOI: 10.1101/2024.11.20.624605
Mechanisms of growth-dependent regulation of the Gin4 kinase
Update in
-
Mechanisms of growth-dependent regulation of the Gin4 kinase.Mol Biol Cell. 2025 Aug 1;36(8):ar94. doi: 10.1091/mbc.E24-12-0568. Epub 2025 Jun 4. Mol Biol Cell. 2025. PMID: 40464802
Abstract
Cell cycle progression is dependent upon cell growth. Cells must therefore translate growth into a proportional signal that can be used to determine when there has been sufficient growth for cell cycle progression. In budding yeast, the protein kinase Gin4 is required for normal control of cell growth and undergoes gradual hyperphosphorylation and activation that are dependent upon growth and proportional to the extent of growth, which suggests that Gin4 could function in mechanisms that measure cell growth. However, the molecular mechanisms that drive hyperphosphorylation of Gin4 are poorly understood. Here, we used biochemical reconstitution and genetic analysis to test hypotheses for the mechanisms that drive phosphorylation of Gin4. We ruled out a previous model in which phosphatidylserine delivered to sites of plasma membrane growth binds Gin4 to initiate autophosphorylation. Instead, we show that Elm1, a homolog of the mammalian Lkb1 tumor suppressor kinase, is sufficient to promote hyperphosphorylation of Gin4 in vitro, likely via initiation of Gin4 autophosphorylation. Furthermore, we show that casein kinase I is required for growth-dependent hyperphosphorylation of Gin4 and also for normal regulation of Elm1. Together, these discoveries lead to new insight into mechanisms that link cell cycle progression to cell growth.
Figures
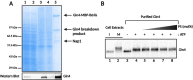

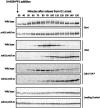


References
-
- Babu P., Bryan J.D., Panek H.R., Jordan S.L., Forbrich B.M., Kelley S.C., Colvin R.T., and Robinson L.C.. 2002. Plasma membrane localization of the Yck2p yeast casein kinase 1 isoform requires the C-terminal extension and secretory pathway function. Journal of Cell Science. 115:4957–4968. doi: 10.1242/jcs.00203. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
