This is a preprint.
Molecular Polymorphism of tau aggregates in Pick's disease
- PMID: 39605720
- PMCID: PMC11601565
- DOI: 10.1101/2024.11.19.624253
Molecular Polymorphism of tau aggregates in Pick's disease
Update in
-
Molecular polymorphism of tau aggregates in Pick's disease.Neurobiol Dis. 2025 Sep 14;216:107104. doi: 10.1016/j.nbd.2025.107104. Online ahead of print. Neurobiol Dis. 2025. PMID: 40957494
Abstract
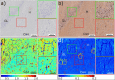
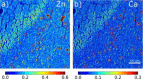
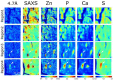
Tau protein plays a central role in many neuropathies. The trajectory by which tau spreads through neural networks is disease-specific but the events driving progression are unknown. This is due in part to the challenge of characterizing tau aggregates in situ. We address that challenge using in situ micro-x-ray diffraction (µXRD) and micro-X-ray fluorescence (μXRF) to examine tau lesions in the brain of a 79-year-old male with dementia. Neuropathological examination revealed classical forms of tau in the hippocampal formation: extensive Pick bodies in the granular layer; modest numbers of neurofibrillary tangles and dystrophic neurites in the CA4 and hilus. µXRD indicated that Pick bodies are low in fibril content, whereas neurofibrillary lesions within adjacent tissue exhibit far greater density of fibrillar tau. μXRF demonstrated elevated levels of zinc, calcium and phosphorous in all tau-containing lesions whereas sulfur deposition was greatest in lesions exhibiting high fibrillar content. Correlation of lesion morphology with anatomical localization, tau fibrillation and differential elemental accumulation suggests tau fibrils generate biochemically distinct microenvironments that influence lesion morphology, tau seed formation and spreading.
Keywords: Biological Sciences; Biophysics and Computational Biology; neurodegenerative disease; tau; x-ray fluorescence microscopy; x-ray microdiffraction.
Conflict of interest statement
Competing Interest Statement: There are no competing interests.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
