This is a preprint.
PrP turnover in vivo and the time to effect of prion disease therapeutics
- PMID: 39605733
- PMCID: PMC11601496
- DOI: 10.1101/2024.11.12.623215
PrP turnover in vivo and the time to effect of prion disease therapeutics
Abstract
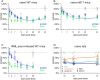
PrP lowering is effective against prion disease in animal models and is being tested clinically. Therapies in the current pipeline lower PrP production, leaving pre-existing PrP to be cleared according to its own half-life. We hypothesized that PrP's half-life may be a rate-limiting factor for the time to effect of PrP-lowering drugs, and one reason why late treatment of prion-infected mice is not as effective as early treatment. Using isotopically labeled chow with targeted mass spectrometry, as well as antisense oligonucleotide treatment followed by timed PrP measurement, we estimate a half-life of 5-6 days for PrP in the brain. PrP turnover is not affected by over- or under-expression. Mouse PrP and human PrP have similar turnover rates measured in wild-type or humanized knock-in mice. CSF PrP appears to mirror brain PrP in real time in rats. PrP is more readily quantifiable in colon than in other peripheral organs, and appears to have a shorter half-life in colon than in brain. Our data may inform the design of both preclinical and clinical studies of PrP-lowering drugs.
Conflict of interest statement
Competing interests BE and CB are employees of IQ Proteomics. ABS is an employee of Charles River Laboratories. NO is an employee and shareholder of Gate Bio. HTZ and BN are employees and shareholders of Ionis Pharmaceuticals. EVM acknowledges speaking fees from Abbvie, Eli Lilly, and Vertex; consulting fees from Alnylam and Deerfield; research support from Eli Lilly, Gate Bio, Ionis, and Sangamo. SMV acknowledges speaking fees from Abbvie, Biogen, Eli Lilly, Illumina, and Ultragenyx; consulting fees from Alnylam and Invitae; research support from Eli Lilly, Gate Bio, Ionis, and Sangamo.
Figures


References
-
- Minikel EV, Zhao HT, Le J, O’Moore J, Pitstick R, Graffam S, Carlson GA, Kavanaugh MP, Kriz J, Kim JB, Ma J, Wille H, Aiken J, McKenzie D, Doh-Ura K, Beck M, O’Keefe R, Stathopoulos J, Caron T, Schreiber SL, Carroll JB, Kordasiewicz HB, Cabin DE, Vallabh SM. Prion protein lowering is a disease-modifying therapy across prion disease stages, strains and endpoints. Nucleic Acids Res. 2020. Aug 10; - PMC - PubMed
-
- Raymond GJ, Zhao HT, Race B, Raymond LD, Williams K, Swayze EE, Graffam S, Le J, Caron T, Stathopoulos J, O’Keefe R, Lubke LL, Reidenbach AG, Kraus A, Schreiber SL, Mazur C, Cabin DE, Carroll JB, Minikel EV, Kordasiewicz H, Caughey B, Vallabh SM. Antisense oligonucleotides extend survival of prion-infected mice. JCI Insight. 2019. 30;5. - PMC - PubMed
-
- Vallabh SM, Minikel EV, Schreiber SL, Lander ES. Towards a treatment for genetic prion disease: trials and biomarkers. Lancet Neurol. 2020. Apr;19(4):361–368. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
