Pronounced electronic modulation of geometrically-regulated metalloenediyne cyclization
- PMID: 39605870
- PMCID: PMC11591729
- DOI: 10.1039/d4sc05396f
Pronounced electronic modulation of geometrically-regulated metalloenediyne cyclization
Erratum in
-
Correction: Pronounced electronic modulation of geometrically-regulated metalloenediyne cyclization.Chem Sci. 2025 Jul 10;16(29):13543-13546. doi: 10.1039/d5sc90147b. eCollection 2025 Jul 23. Chem Sci. 2025. PMID: 40656528 Free PMC article.
Abstract
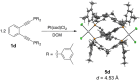
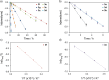
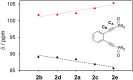
Using a diverse array of thermally robust phosphine enediyne ligands (dxpeb, X = Ph, Ph-pOCH3, Ph-pCF3, Ph-m 2CH3, Ph-m 2CF3, iPr, Cy, and t Bu) a novel suite of cisplatin-like Pt(ii) metalloenediynes (3, Pt(dxpeb)Cl2) has been synthesized and represents unique electronic perturbations on thermal Bergman cyclization kinetics. Complexes 3e (Ph-m 2CF3) and 3f (iPr) are the first of this structure type to be crystallographically characterized with inter alkyne termini distances (3e: 3.13 Å; 3f: 3.10 Å) at the lower end of the widely accepted critical distance range within which enediynes should demonstrate spontaneous ambient temperature cyclization. Despite different electronic profiles, these metalloenediynes adopt a rigid, uniform structure suggesting complexes of the form Pt(dxpeb)Cl2 have orthogonalized geometric and electronic contributions to thermal Bergman cyclization. Kinetic activation parameters determined using 31P NMR spectroscopy highlight the dramatic reactivity and thermal tunability of these complexes. At room temperature, the half-life (t 1/2) of cyclization spans a range of ∼35 hours and for the aryl phosphine derivatives, cycloaromatization rates are 10-30 times faster for complexes with electron donating substituents (3b: Ph-pOCH3; 3d: Ph-m 2CH3) compared to those with electron withdrawing substituents (3c: Ph-pCF3; 3e: Ph-m 2CF3). Computational interrogation of the aryl phosphine metalloenediynes 3a-3e reveals that the origin of this precise electronic control derives from electronic withdrawing group-mediated alkyne carbon polarization that amplifies coulombic repulsion increasing the cyclization barrier height. Additionally, mixing between the in-plane π-orbitals and the phosphine aryl ring system is pronounced for complexes with electron donating substituents which stabilizes the developing C-C bond and lowers the activation barrier. This π-orbital mixing is negligible however, for complexes with electron withdrawing substituents due to an energetic mismatch of the orbital systems. Overall, this work demonstrates that for geometrically rigid frameworks, even remote enediyne functionalization can have pronounced effects on activation barrier.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures









Similar articles
-
Transition-State Stabilization by Secondary Orbital Interactions between Fluoroalkyl Ligands and Palladium During Reductive Elimination from Palladium(aryl)(fluoroalkyl) Complexes.ACS Catal. 2023 Oct 6;13(19):12810-12825. doi: 10.1021/acscatal.3c02648. Epub 2023 Sep 18. ACS Catal. 2023. PMID: 40756021 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Can a Liquid Biopsy Detect Circulating Tumor DNA With Low-passage Whole-genome Sequencing in Patients With a Sarcoma? A Pilot Evaluation.Clin Orthop Relat Res. 2025 Jan 1;483(1):39-48. doi: 10.1097/CORR.0000000000003161. Epub 2024 Jun 21. Clin Orthop Relat Res. 2025. PMID: 38905450
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
References
-
- Edo K. Mizugaki M. Koide Y. Seto H. Furihata K. Otake N. Ishida N. The Structure of Neocarzinostatin Chromophore Possessing a Novel Bicyclo-[7,3,0]Dodecadiyne System. Tetrahedron Lett. 1985;26:331–334. doi: 10.1016/S0040-4039(01)80810-8. - DOI
-
- Golik J. Clardy J. Dubay G. Groenewold G. Kawaguchi H. Konishi M. Krishnan B. Ohkuma H. Saitoh K. Doyle T. W. Esperamicins, A Novel Class of Potent Antitumor Antibiotics. 2. Structure of Esperamicin X. J. Am. Chem. Soc. 1987;109:3461–3462. doi: 10.1021/ja00245a048. - DOI
-
- Golik J. Dubay G. Groenewold G. Kawaguchi H. Konishi M. Krishnan B. Ohkuma H. Saitoh K. Doyle T. W. Esperamicins, A Novel Class of Potent Antitumor Antibiotics. 3. Structures of Esperamicins A1, A2, and A1b. J. Am. Chem. Soc. 1987;109:3462–3464. doi: 10.1021/ja00245a049. - DOI
-
- Lee M. D. Dunne T. S. Chang C. C. Ellestad G. A. Siegel M. M. Morton G. O. McGahren W. J. Borders D. B. Calichemicins, A Novel Family of Antitumor Antibiotics. 2. Chemistry and Structure of Calichemicin g1. J. Am. Chem. Soc. 1987;109:3466–3468. doi: 10.1021/ja00245a051. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

