Characterization of the Retinal Phenotype Using Multimodal Imaging in Novel Compound Heterozygote Variants of CYP2U1
- PMID: 39605873
- PMCID: PMC11599445
- DOI: 10.1016/j.xops.2024.100618
Characterization of the Retinal Phenotype Using Multimodal Imaging in Novel Compound Heterozygote Variants of CYP2U1
Abstract
Purpose: To report the retinal phenotype in 2 patients simulating type 2 macular telangiectasis with new variants in CYP2U1 implicated in hereditary spastic paraplegia type 56 (HSP 56).
Design: Cross sectional case series study.
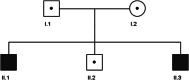
Participants: Five members of a non-consanguineous family (parents and 3 male children) were investigated.
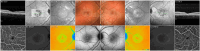
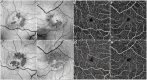
Methods: All family members underwent a full ophthalmic evaluation and multimodal retinal imaging. Two family members demonstrating retinal anomalies underwent additional OCT angiography, dual wavelength autofluorescence and fluorescence lifetime imaging ophthalmoscopy, kinetic perimetry, fundus-correlated microperimetry, electroretinography, and electro-oculography. Whole-exome sequencing was performed in all 5 family members.
Main outcome measures: To characterize the retinal phenotype in affected patients with variants in CYP2U1, using multimodal imaging: dual-wavelength autofluorescence, fluorescence lifetime, OCT angiography.
Results: The 2 siblings with compound heterozygous novel variants c.452C>T; p.(Pro151Leu), c.943C>T; p.(Gln315Ter) in CYP2U1 demonstrated parafoveal loss of retinal transparency and hyperreflectivity to blue light, redistribution of macular pigment to the parafoveal edge, photoreceptor loss, and fluorescence lifetime imaging ophthalmoscopy anomalies: a pattern compatible with that seen in macular telangiectasia type 2 (MacTel). One had manifest neurological abnormalities since early childhood; the second had no neurological abnormalities. Each parent and the third sibling were heterozygous for 1 variant and were neurologically and ophthalmically normal.
Conclusions: These CYP2U1 variants are associated with a retinal phenotype very similar to that otherwise specific for MacTel, suggestive of possible links in the etiology and pathogenesis of these diseases.
Financial disclosures: The author(s) have no proprietary or commercial interest in any materials discussed in this article.
Keywords: CYP2U1; Hereditary Spastic Paraplegia type 56; MacTel; Maculopathy; Multimodal imaging.
© 2024 by the American Academy of Ophthalmology.
Figures



References
-
- Chuang S.S., Helvig C., Taimi M., et al. CYP2U1, a novel human thymus- and brain-specific cytochrome P450, catalyzes omega- and (omega-1)-hydroxylation of fatty acids. J Biol Chem. 2004;279:6305–6314. - PubMed
LinkOut - more resources
Full Text Sources

