ReCLAIM-2: A Randomized Phase II Clinical Trial Evaluating Elamipretide in Age-related Macular Degeneration, Geographic Atrophy Growth, Visual Function, and Ellipsoid Zone Preservation
- PMID: 39605874
- PMCID: PMC11599447
- DOI: 10.1016/j.xops.2024.100628
ReCLAIM-2: A Randomized Phase II Clinical Trial Evaluating Elamipretide in Age-related Macular Degeneration, Geographic Atrophy Growth, Visual Function, and Ellipsoid Zone Preservation
Abstract
Objective: This study evaluated the safety and efficacy of elamipretide in dry age-related macular degeneration (AMD) with noncentral geographic atrophy (GA).
Design: ReCLAIM-2 was a prospective, phase II, randomized, placebo-controlled, double-masked, multicenter trial (NCT03891875).
Subjects: Patients aged ≥55 years with ≥1 eye with dry AMD with GA were enrolled.
Methods: Administration of daily subcutaneous elamipretide 40 mg was investigated in subjects for 48 weeks followed by a 4-week follow-up period.
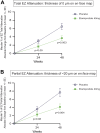
Main outcome measures: The primary efficacy end points were the mean change from baseline (BL) in low-luminance best-corrected visual acuity (LL BCVA) and the change in square root (Sqrt) converted GA area from BL as measured by OCT. Additional predefined end points included ellipsoid zone (EZ) integrity preservation assessment and categorical changes in LL BCVA. The primary safety end point was the incidence and severity of adverse events.
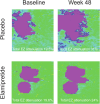
Results: Of the 176 patients randomized, there were 117 and 59 patients in the elamipretide and placebo groups, respectively. Although elamipretide did not meet statistical significance for the primary end points (mean change in LL BCVA and mean change in Sqrt converted GA area), elamipretide produced a 43% reduction in the mean progression from BL in the macular percentage of total EZ attenuation/loss (i.e., complete loss of EZ band; nominal P = 0.0034) and 47% reduction in the mean progression of macular percentage of partial EZ attenuation/degradation (i.e., EZ-retinal pigment endothelium thickness of ≤20 microns; nominal P = 0.0040) versus placebo at week 48. Elamipretide treatment was also associated with significantly more patients experiencing a ≥10 letter gain in LL BCVA versus placebo (14.6% vs. 2.1%; nominal P = 0.0404). Adverse events were reported in 86% of those receiving elamipretide and 71% of the placebo group with the most common events being injection site reactions (e.g., pruritus, injection site pain, bruising, and erythema).
Conclusions: While the primary end points were not met in this phase II study, elamipretide treatment was associated with a slowing of progressive EZ degradation/loss, a surrogate for photoreceptor damage. These findings have important clinical relevance since EZ attenuation/photoreceptor loss precedes and predicts the progressive pathological changes associated with vision loss and AMD. The EZ attenuation/loss end point will serve as the regulatory approved primary end point in the elamipretide phase III clinical development program.
Financial disclosures: Proprietary or commercial disclosure may be found in the Footnotes and Disclosures at the end of this article.
Keywords: Age-related macular degeneration; Elamipretide; Ellipsoid zone; Geographic atrophy; Visual acuity.
© 2024 by the American Academy of Ophthalmology.
Figures


References
-
- Rein D.B., Wittenborn J.S., Zhang X., et al. Forecasting age-related macular degeneration through the year 2050: the potential impact of new treatments. Arch Ophthalmol. 2009;127:533–540. - PubMed
-
- Chakravarthy U., Bailey C.C., Johnston R.L., et al. Characterizing disease burden and progression of geographic atrophy secondary. To age-related macular degeneration. Ophthalmology. 2018;125:842–849. - PubMed
-
- Holz F.G., Strauss E.C., Schmitz-Valckenberg S., et al. Geographic atrophy: clinical features and potential therapeutic approaches. Ophthalmology. 2014;121:1079–1091. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

