Cost-effectiveness analysis of trifluridine/tipiracil combined with bevacizumab vs. monotherapy for third-line treatment of colorectal cancer
- PMID: 39606076
- PMCID: PMC11599266
- DOI: 10.3389/fpubh.2024.1465898
Cost-effectiveness analysis of trifluridine/tipiracil combined with bevacizumab vs. monotherapy for third-line treatment of colorectal cancer
Abstract
Background: The combination of trifluridine/tipiracil (FTD/TPI) and bevacizumab has demonstrated promising efficacy and safety in the treatment of colorectal cancer (CRC). This study aims to evaluate the cost-effectiveness of trifluridine/tipiracil combined with bevacizumab vs. trifluridine/tipiracil monotherapy as a third-line treatment regimen for colorectal cancer within the Chinese healthcare system, providing an economic basis for clinical application.
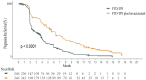
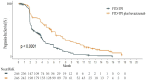
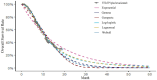
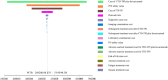
Methods: Based on data from the SUNLIGHT Phase III clinical trial, a dynamic Markov model was constructed with a cycle length of 4 weeks and a simulation duration of 10 years. Direct medical costs and quality-adjusted life years (QALYs) were calculated. The incremental cost-effectiveness ratio (ICER) was compared with the willingness-to-pay threshold (WTP = ¥268,200.00/QALY) to assess the economic viability of the treatment regimen. One-way sensitivity analysis and probabilistic sensitivity analysis were conducted to verify the robustness of the model results.
Results: The cost of trifluridine/tipiracil combined with bevacizumab treatment (¥838,492.74) was higher than that of trifluridine/tipiracil monotherapy (¥357,396.97), with greater health benefits (2.45 QALYs vs. 1.54 QALYs). The ICER was ¥527,577.36/QALY, exceeding the willingness-to-pay threshold. One-way sensitivity analysis indicated that drug costs and utility values during the progression-free period significantly impacted model outputs. Probabilistic sensitivity analysis further confirmed the robustness of the results, showing that at a willingness-to-pay threshold of ¥494,000.00, the probability of the combined treatment being cost-effective was 50%.
Conclusion: Trifluridine/tipiracil combined with bevacizumab, as a third-line treatment for colorectal cancer, does not have a cost-effectiveness advantage compared to trifluridine/tipiracil monotherapy in economic evaluations.
Keywords: Markov model; bevacizumab; colorectal cancer; cost-effectiveness analysis; trifluridine/tipiracil.
Copyright © 2024 Huang, Chen, Gu and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- He B, Liu JP, Sun H, Yang YF, Li MZ, Li PP, et al. . Guidelines for traditional Chinese medicine intervention after conventional Western medicine treatment for stage I-III colorectal Cancer. Chin J Exp Tradit Med Formulae. (2023) 29:1–9.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

