Invention and characterization of a systemically administered, attenuated and killed bacteria-based multiple immune receptor agonist for anti-tumor immunotherapy
- PMID: 39606250
- PMCID: PMC11599860
- DOI: 10.3389/fimmu.2024.1462221
Invention and characterization of a systemically administered, attenuated and killed bacteria-based multiple immune receptor agonist for anti-tumor immunotherapy
Abstract
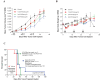
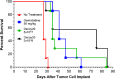
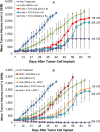
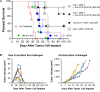
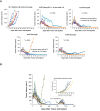
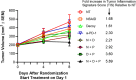
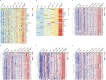
Activation of immune receptors, such as Toll-like (TLR), NOD-like (NLR) and Stimulator of Interferon Genes (STING) is critical for efficient innate and adaptive immunity. Gram-negative bacteria (G-NB) contain multiple TLR, NOD and STING agonists. Potential utility of G-NB for cancer immunotherapy is supported by observations of tumor regression in the setting of infection and Coley's Toxins. Coley reported that intravenous (i.v.) administration was likely most effective but produced uncontrollable toxicity. The discovery of TLRs and their agonists, particularly the potent TLR4 agonist lipopolysaccharide (LPS)-endotoxin, comprising ~75% of the outer membrane of G-NB, suggests that LPS may be both a critical active ingredient and responsible for dose-limiting i.v. toxicity of G-NB. This communication reports the production of killed, stabilized, intact bacteria products from non-pathogenic G-NB with ~96% reduction of LPS-endotoxin activity. One resulting product candidate, Decoy10, was resistant to standard methods of cell disruption and contained TLR2,4,8,9, NOD2 and STING agonist activity. Decoy10 also exhibited reduced i.v. toxicity in mice and rabbits, and a largely uncompromised ability to induce cytokine and chemokine secretion by human immune cells in vitro, all relative to unprocessed, parental bacterial cells. Decoy10 and a closely related product, Decoy20, produced single agent anti-tumor activity or combination-mediated durable regression of established subcutaneous, metastatic or orthotopic colorectal, hepatocellular (HCC), pancreatic, and non-Hodgkin's lymphoma (NHL) tumors in mice, with induction of both innate and adaptive immunological memory (syngeneic and human tumor xenograft models). Decoy bacteria combination-mediated regressions were observed with a low-dose, oral non-steroidal anti-inflammatory drug (NSAID), anti-PD-1 checkpoint therapy, low-dose cyclophosphamide (LDC), and/or a targeted antibody (rituximab). Efficient tumor eradication was associated with plasma expression of 15-23 cytokines and chemokines, broad induction of cytokine, chemokine, innate and adaptive immune pathway genes in tumors, cold to hot tumor inflammation signature transition, and required NK, CD4+ and CD8+ T cells, collectively demonstrating a role for both innate and adaptive immune activation in the anti-tumor immune response.
Keywords: TLR agonist; adaptive; anti-cancer; anti-tumor; bacteria; immunotherapy; innate; toll-like receptor.
Copyright © 2024 Newman.
Conflict of interest statement
MN is an employee, director and stockholder of Indaptus Therapeutics, Inc.
Figures
References
-
- Murphy K, Weaver C, Berg L, Janeway C. Janeway’s immunobiology. 10th edition. New York, NY: W.W. Norton and Company; (2022). 43 p.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

