This is a preprint.
Variants in NR6A1 cause a novel oculo-vertebral-renal (OVR) syndrome
- PMID: 39606382
- PMCID: PMC11601759
- DOI: 10.1101/2024.11.09.24316578
Variants in NR6A1 cause a novel oculo-vertebral-renal (OVR) syndrome
Update in
-
Variants in NR6A1 cause a novel oculo vertebral renal syndrome.Nat Commun. 2025 Jul 3;16(1):6111. doi: 10.1038/s41467-025-60574-y. Nat Commun. 2025. PMID: 40610405 Free PMC article.
Abstract
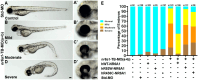
Colobomatous microphthalmia is a potentially blinding congenital ocular malformation that can present either in isolation or together with other syndromic features. Despite a strong genetic component to disease, many cases lack a molecular diagnosis. We describe a novel autosomal dominant oculo-vertebral-renal (OVR) syndrome in six independent families characterized by colobomatous microphthalmia, missing vertebrae and congenital kidney abnormalities. Genome sequencing identified six rare variants in the orphan nuclear receptor gene NR6A1 in these families. We performed in silico, cellular and zebrafish experiments to demonstrate the NR6A1 variants were pathogenic or likely pathogenic for OVR syndrome. Knockdown of either or both zebrafish paralogs of NR6A1 results in abnormal eye and somite development, which was rescued by wild-type but not variant NR6A1 mRNA. Illustrating the power of genomic ascertainment in medicine, our study establishes NR6A1 as a critical factor in eye and vertebral development and a pleiotropic gene responsible for OVR syndrome.
Figures



References
-
- Williamson K.A., and FitzPatrick D.R. (2014). The genetic architecture of microphthalmia, anophthalmia and coloboma. Eur. J. Med. Genet. 57, 369–380. - PubMed
-
- Chang L., Blain D., Bertuzzi S., and Brooks B.P. (2006). Uveal coloboma: clinical and basic science update. Curr. Opin. Ophthalmol. 17, 447–470. - PubMed
-
- Patel A., and Sowden J.C. (2019). Genes and pathways in optic fissure closure. Semin. Cell Dev. Biol. 91, 55–65. - PubMed
-
- ALSomiry A.S., Gregory-Evans C.Y., and Gregory-Evans K. (2019). An update on the genetics of ocular coloboma. Hum. Genet. 138, 865–880. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
